Introduction
Electronic health records (EHRs) are central to care delivery, with nearly every healthcare facility in the United States using this technology.1 EHRs are powerful tools that have digitized medicine and, in many circumstances, have provided a structured format for communicating critical information between different healthcare team members. For example, computerized provider order entry (CPOE) enables prescribers to place medication, laboratory, and radiology orders that can be electronically transmitted to other members of the healthcare team in a consistently structured format which may help reduce variability that can cause patient safety issues.2,3 CPOE typically contains structured fields in which medication-specific information (e.g., dose, frequency, route) can be entered. Aside from CPOE, there is other EHR functionality that enables structured documentation of allergies, height, and weight. This information then serves to guide a host of services from dietary to medication dosing.
While structured fields bring standardization and consistency to how information is collected, documented, and communicated, they may be insufficient at providing the full context of what is needed for safe and effective care delivery. For example, when placing a medication order, a prescriber may wish to add information to complement the structured order, such as instructing the nurse to give a medication only if a certain condition has been met (e.g., the patient has completed hemodialysis, one hour before a procedure/test is to begin).4 It is difficult to design EHR interfaces that provide structured fields and information categories for all the different types of information a provider may wish to communicate. For this reason, many EHR functions contain a field that enables free text so providers can communicate additional contextual information. This free-text information is known by various names in clinical practice and the research literature, including special instructions, special comments, order comments, or order instructions.
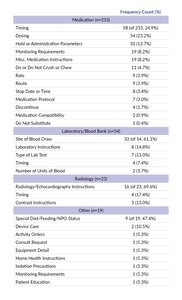
Patient safety issues have been associated with the use of free text—especially in CPOE systems—due to information that is inconsistent or contradictory with other structured parts of a CPOE order.5–8 One study showed that 13.5% of all prescriptions with special instructions included discrepancies that could lead to adverse drug events, including underdosing, incorrect route, or ignoring dose escalation or tapering.9 Special instructions through free-text fields may never be received if different healthcare team member roles have different levels of access to such fields, or if they are not plainly visible in the order.10,11 Discrepancies between free-text instructions and the order they are associated with could potentially be intercepted by pharmacists at the dispensing stage when pharmacists receive the free-text information. One study found 34.3% of information about how to fill a prescription that was intended for pharmacists could have resulted in patient harm if it were missed by the pharmacist.12 Another consequence of using free-text to complement orders is when incomplete or unclear instructions about medication usage are given to pharmacy personnel, they may make inaccurate assumptions and delete directions or rewrite them inaccurately without checking back in with the prescriber.11 However, clarifying the order with the prescriber can cause workflow inefficiencies and delay medication delivery to the patient.9
One study looking at medication-related patient safety event (PSE) reports showed that an electronic medication administration display that was confusing, cluttered, or inaccurate resulted in missed special instructions (e.g., note entered by ordering provider for medication to be administered only after dialysis, but special instructions not seen by administering team), which may also contribute to errors when receiving the free-text information.13 Issues may also arise when special instructions about laboratory orders are entered into a free-text field in the EHR order form but that information does not get transferred to the laboratory information systems interface. This issue can result in delays if laboratory offices have to call the clinician’s offices for clarification, missed laboratory orders, tests performed on the wrong date, and a disruption of laboratory workflows.14
Developing a deeper understanding of the conditions under which free text is used to complement orders in the EHR may provide an opportunity to optimize EHR interfaces and healthcare facility processes and procedures. For example, the frequent use of free-text comments in prescriptions may suggest a lack of suitable structured functions within the EHR.5 In this study, we seek to develop a deeper understanding of when free text is used in EHR orders through the analysis of PSE reports. Based on our analysis, we provide EHR design strategies and policies and protocols to address patient safety issues associated with free text to enable safer and more resilient care delivery.
Methods
Data Source and Selection
We analyzed PSE reports submitted to the Pennsylvania Patient Safety Reporting System (PA-PSRS)[1] between January 1, 2021, and December 31, 2022. All nonfederal, acute care facilities in Pennsylvania are required to report PSEs through PA-PSRS. To identify PSE reports that likely referred to safety issues connected to free-text entries and EHR ordering, we searched the free-text event details of the PSE and its other relevant free-text and structured fields with the following keywords: “special instruction(s),” “order instruction(s),” “order comment(s),” and “special comment(s).” Additionally, we expanded the search to allow for up to five words between the first and second words of each (i.e., between “special” or “order” and “instruction[s]” or “comment[s]”) to capture a wider array of relevant phrasing. This search resulted in 847 reports; however, 182 (21.5%) reports were submitted by one facility.
To prevent oversampling from one facility, we randomly selected a maximum of 42 reports (5% of total reports retrieved) from each facility. Based on this sampling strategy, a total of 677 reports from 129 facilities were reviewed. Of the 677 reports reviewed, 329 (48.6%) reports were included in our analysis. Reports were included if special instructions, order instructions, order comments, or special comments were mentioned in the PSE report and the special instructions, order instructions, order comments, or special comments contributed to the safety issue. In some cases, it was not entirely clear whether the use of a term such as “special instructions” in the PSE report (e.g., “staff unaware of special instructions for transport”) referred to a free-text comment in the EHR or a more general guideline regarding the need for special handling/treatment; in these situations, we used best judgment to determine whether reports met inclusion criteria.
Coding Taxonomy and Analysis Methods
The PSE fields of “event description” and “event recommendation” were qualitatively analyzed using a grounded theory approach and additional structured report categories were analyzed for further context. Examples of structured report categories included Event Type, Event Subtype, Care Area Type, and Patient Status. Our coding taxonomies were iteratively developed.
Reports that met the inclusion criteria were further reviewed. Each report was assigned a single code for each of the criterion described below:
-
General care process: Describes the care process associated with the safety issue (see Table 1 for definitions of general care processes).
- Medication class: If the report was categorized into the Medication category, the specific medication that was involved in the special instruction issue was identified either in the free-text description or the required PA-PSRS “medication prescribed” name field. If multiple medications were noted within one report, a primary medication was identified. A clinical expert then classified medications into a medication class (see Table 2 for medication classes).
-
Information expressed in the special instructions: Details about what the ordering provider was attempting to communicate within each general care process category (see Table 3 for categories of information expressed under general care processes).
-
Special instructions issue: Specific type of difficulty in accessing, understanding, or interpreting the special instruction (see Table 4 for definitions of types of issues).
-
Department or staff that received or was supposed to act on the special instruction: The specific healthcare staff (e.g., nurse, pharmacy) or department (e.g., laboratory/blood bank, radiology) for which special instructions were intended (see Table 5).
-
Reached the patient: The error related to use or absence of required special instructions impacted the patient’s care.
Coding Process
A physician and human factors subject matter expert independently reviewed and coded all reports, resulting in each report being dually coded. Discrepancies were discussed until consensus was reached.
Results
Below we describe results for the following categories of the 329 reports included in our analysis: general care process, medication class, information expressed in the special instructions, special instructions issue, who or what department received/was supposed to see or act upon the special instructions, and whether the error reached the patient.
General Care Process
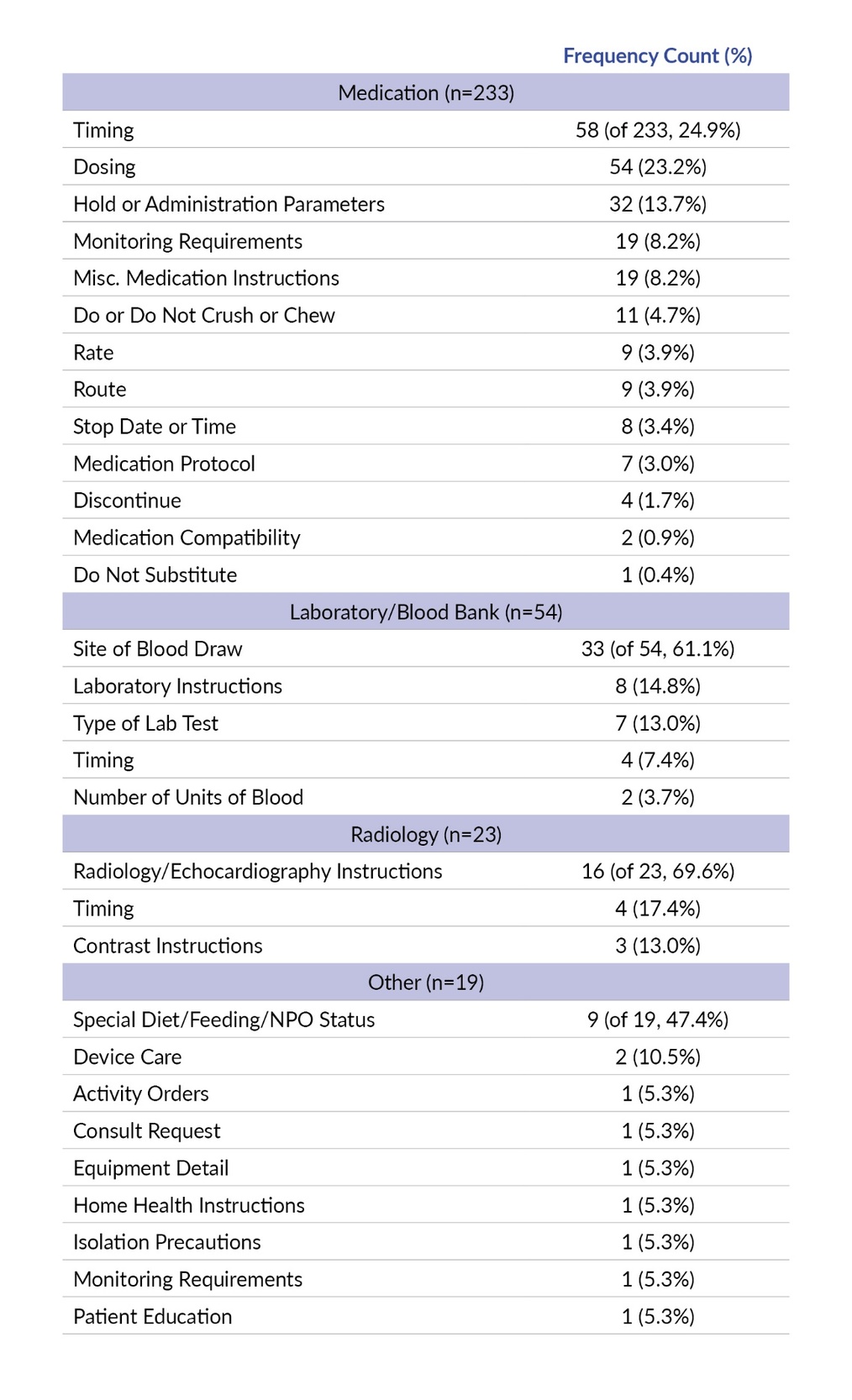
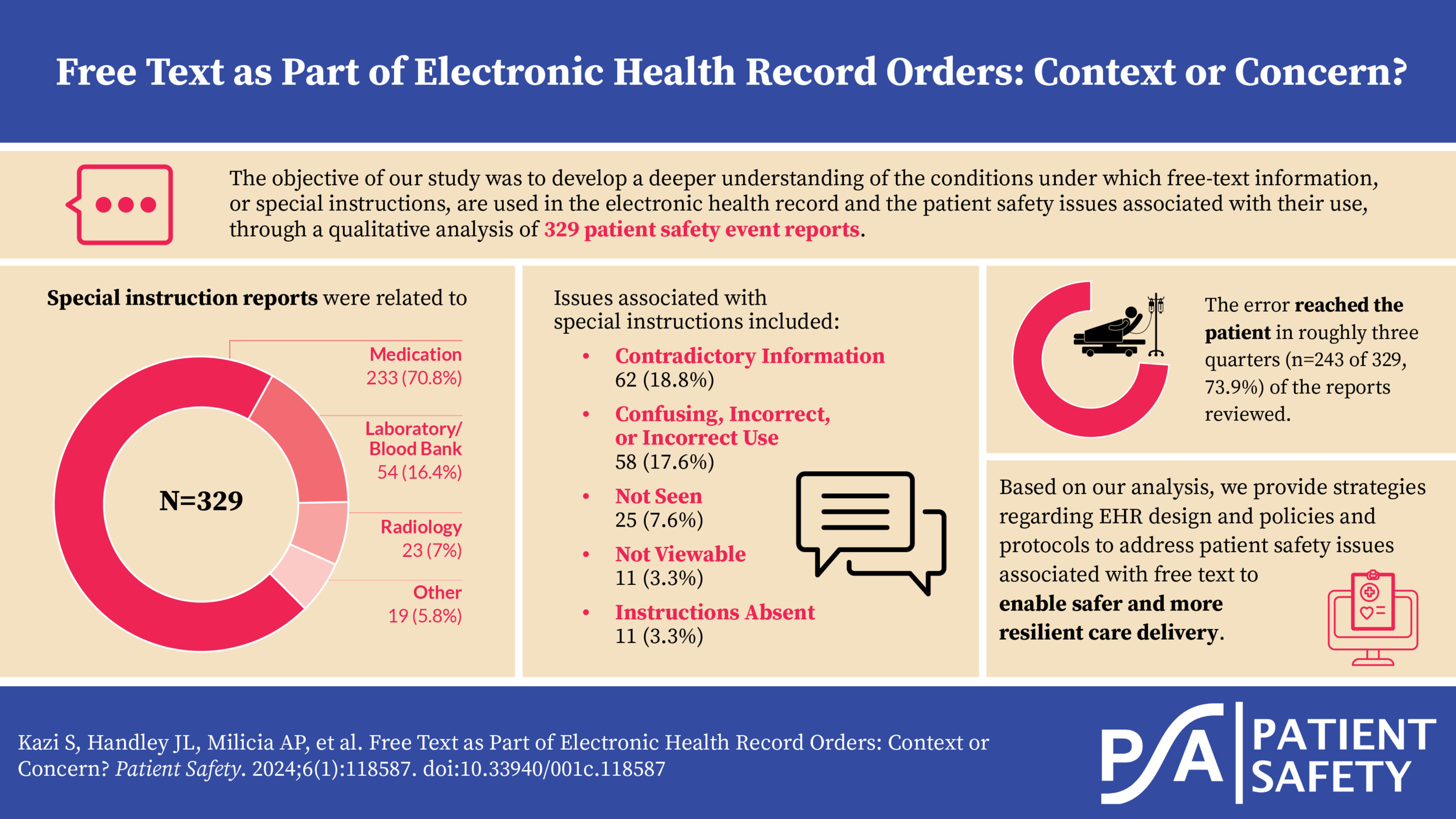
About three-quarters of the special instruction reports were related to Medication (n=233 of 329, 70.8%) followed by Laboratory/Blood Bank (n=54, 16.4%), Radiology (n=23, 7.0%), and Other (n=19, 5.8%) (Table 1).
Medication Class
All special instruction reports in the Medication general care process explicitly stated the medication involved except three reports (N=230), and these medications were categorized into their respective medication classes. The three most frequent medication classes accounting for more than 10% of reports included infectious disease medications (n=51 of 230, 22.2%), antithrombotic/antithrombotic reversal agents (n=32, 13.9%), and nutritional/electrolytes/intravenous (IV) fluids (n=32, 13.9%). A comprehensive table of all medication classes is displayed in Table 2 below.
Information Expressed in the Special Instructions
We analyzed information expressed in the special instructions by the general care process. In the general care process of Medication, roughly a quarter of reports reviewed were related to timing (n=58 of 233, 24.9%) and nearly another quarter were related to dosing (n=54, 23.2%). Most of Laboratory/Blood Bank special instructions information was related to confusion caused by special instructions about the catheter or location on the patient’s body from which the blood was drawn (site of blood draw, n=33 of 54, 61.1%). Many Radiology issues were related to radiology/echocardiography instructions (n=16 of 23, 69.6%). About half of the Other special instructions information was related to a special diet, feeding, or NPO (i.e., nothing by mouth) status (n=9 of 19, 47.4%). A comprehensive table of special instruction information expressed by the general care process is displayed in Table 3 below.
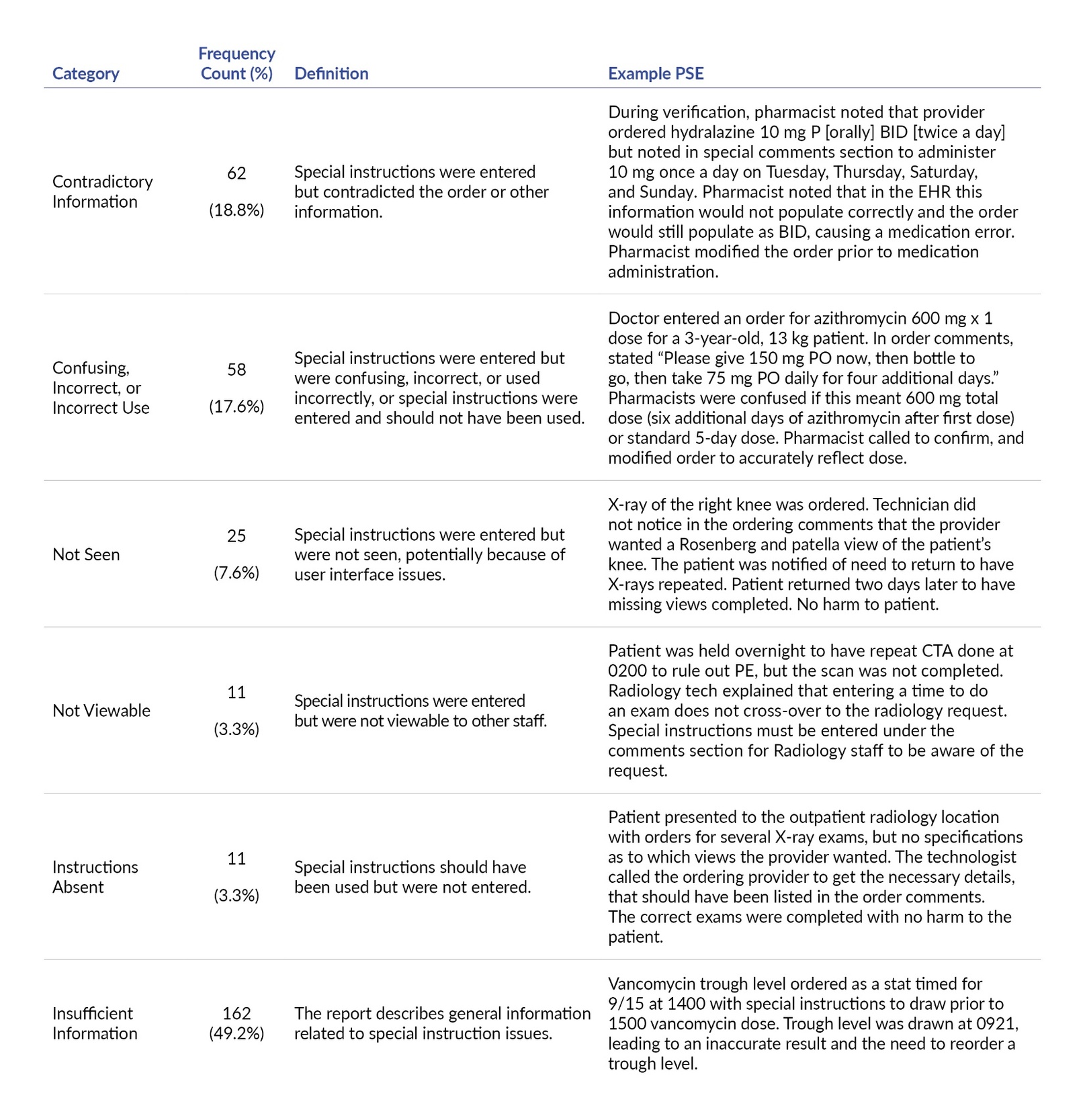
Special Instructions Issue
In approximately one-fifth of reports reviewed (n=62 of 329, 18.8%), the special instructions contradicted other information in the order. The second most frequent issue was special instructions being confusing, incorrect, or used incorrectly (n=58, 17.6%), followed by not seen (n=25, 7.6%), not viewable (n=11, 3.3%), and instructions absent (n=11, 3.3%). There was insufficient information to determine the specific special instructions issue in roughly half of the reports reviewed (n=162, 49.2%) (Table 4).
Who or What Department Received/Was Supposed to See or Act Upon the Special Instructions
Nursing (n=184 of 329, 55.9%) was the role that most frequently received or was supposed to see or act upon the special instructions, followed by pharmacy (n=49, 14.9%), radiology (n=21, 6.4%), laboratory/blood bank (n=20, 6.1%), and other hospital staff (n=12, 3.6%). There was insufficient information in 43 reports (13.1%) to determine who or what department received or was supposed to see or act upon the special instructions (Table 5).
Error Reached the Patient
The error reached the patient in roughly two-thirds (n=243 of 329, 73.9%) of the reports reviewed.
Discussion
Special instructions are frequently used to add context about orders in the EHR but can be associated with patient safety precursor and harm events. We analyzed PSE reports related to special instructions to investigate the types and frequency of medical care processes associated with special instructions, and to understand how their use contributes to patient safety issues. Our analyses provide important insights to guide the use of special instructions and potential redesign of EHR functionality.
Special instructions were most frequently associated with medication orders (specifically dose and timing of the medications) and laboratory orders (specifying the site of blood draws). A recent systematic review found that 9% of pooled patient incidence records about medication errors were associated with harm.15 Fifty-eight percent of these medication errors occurred at the stage of medication prescribing. Although CPOE can help in reducing some types of medication errors, such errors remain prevalent despite the adoption of electronic prescribing, dispensing, and administration.16 Further, many studies on medication errors typically analyze the information in structured fields such as dose, time, and route, and potentially do not count the contribution of confusion about information contained in free-text fields such as special instructions to the overall medication error rate. In fact, special instructions are often used as a workaround to give medication directions that are not present in current structured fields on the CPOE.17–19 This workaround suggests an opportunity to reevaluate the current design of medication and laboratory orders to add fields to specify the context provided by special instructions.
It was noteworthy that the special instructions in almost a fifth of the reports we reviewed contradicted the information contained in other places in the order. This contradiction has also been found in previous literature, contributing to confusion in interpreting special instructions.20 In addition, special instructions were also associated with other problems, including containing incorrect information and not being viewable or visible, which also supports previous literature about usability challenges of special instructions.21 Nurses were the intended recipients of over half of the special instructions, followed by pharmacists. Previous research already indicates the importance of physician-nurse communication on timely and accurate administration of the care plan, as well as challenges in using special instructions to communicate many aspects of medications and the care plan.19,22
Taken together, these findings suggest that most special instructions may be written by prescribers to alter or supplement the available medication order fields in CPOE and to try to alert nurses and pharmacists to medication administration and dispensing that is different from usual practice. The ubiquitous use of special instructions as a workaround is an indication of a system failure related to communication. Special instructions may also reflect poor usability of CPOE to match clinical workflows, specifically limited capabilities of CPOE to adjust to dynamic changes in the patient’s status or medication availability that may ultimately influence medication administration, or challenges that healthcare teams face in communicating with each other about the patient’s medications and care plan.21
Strategies to Address Special Instruction Patient Safety Issues
There are several strategies that follow from our analysis of PSE reports. These strategies can broadly be described as EHR-based strategies and policy/protocol-based strategies.
EHR-Based Strategies
Assess whether free text could be transitioned into creation of new structured fields: Facilities should routinely investigate the information content of special instructions (e.g., medications, labs, blood tests), and consider adding new structured options to the EHR to integrate this information into the ordering workflow and/or clearly describing standard organizational processes for handling situations in which structured fields do not support ordering.
Determine whether free text should be used if an alternate structured field already exists: The design of EHRs may make it difficult for physicians to find structured fields they intend to use to input specific types of information. Facilities should review the label of existing structured fields to ensure they are easy to understand and find so that structured fields are used as designed.
Simplify ordering interfaces: Some EHRs contain two or more free-text entry fields and the purpose of these fields may be unclear to the ordering provider. Facilities should consider removing unnecessary fields and clearly explaining the purpose of different types of free-text entry fields so providers know which fields to use for what purpose.
Evaluate the entire ordering process: There were several special instruction issues associated with the nurse or pharmacist not seeing the special instructions or the information not being available in their view of the EHR. Facilities should conduct end-to-end usability testing which entails analyzing the workflow of ordering from the point of order entry by the clinician to execution of the order. Each EHR interface involved in this process across all stakeholders should be analyzed to determine whether critical ordering, dispensing, and administration information is appropriately available.
The following principles should be used to guide this type of testing:
-
Providers should be able to see which team members will be able to see the special instructions.
-
When special instructions are used, there should be an indicator that they are associated with an order.
-
Special instructions should be easily accessible and viewable (e.g., on the main screen or within a click).
-
The information referenced by the special instructions should be presented concurrently with the special instructions on the screen to avoid memory burden of remembering the details from the instructions.
Policy/Protocol-Based Strategies
Develop shared awareness and expectations for when special instructions should not be used: Facilities should develop protocols regarding conditions for which special instructions should not be used. One example is clinical nuances that require back-and-forth communication between different members of the healthcare team (e.g., deciding when medications should be administered relative to time of hemodialysis). These protocols should be shared with all members of the care team, especially physicians, nurses, and pharmacists, to ensure shared awareness.
Establish synchronous or near-synchronous communication pathways integrated into the EHR: Facilities should institute protocols to guide communication about when electronic, asynchronous communication should be converted into real-time synchronous or near-synchronous communication. For example, there should be pathways in the EHR to quickly obtain feedback or clarification about unclear special instructions for time-sensitive or high-risk orders.
Establish protocols about details included in distinct free-text fields: There may be instances in which multiple free-text fields are necessary for each order. In cases where the use of multiple free-text fields is warranted, facilities should establish clear protocols about what types of information should be included in each field and ensure that data are distinct from each other. In addition, facilities should also establish protocols about which members of the healthcare team can see which types of special instructions.
Limitations
We examined PSE reports which represent a single data source from one state (Pennsylvania), so results may not be generalizable across other data types or states. In addition, because of the limited descriptive information in the PSE reports, we were unable to understand the larger context of the error reported. In some cases, the special instruction may refer to a certain laboratory or blood bank specimen that needed special treatment (e.g., during transport); however, our analysis did not exclude these cases. Finally, we did not follow up with facilities to gather additional information about each specific report.
Conclusions
Special instructions are often intended for nurses and pharmacists, and are frequently used to provide additional context to guide medication administration and dispensing, as well as laboratory and radiology procedures. However, these instructions can lead to errors because they can be contradictory to the medication order, confusing or incorrect, or not be viewable, and may result in patient harm. Human factors principles can be used to design EHR-based strategies and protocols to improve the safety of special instructions.
Notes
This study was approved by the MedStar Health Research Institute institutional review board.
Disclosure
The authors declare that they have no relevant or material financial interests.
About the Authors
Sadaf Kazi is a senior research scientist at the MedStar Health National Center for Human Factors in Healthcare and an associate professor at Georgetown University School of Medicine.
Jessica L. Handley is the associate director of operations at the MedStar Health National Center for Human Factors in Healthcare.
Arianna P. Milicia is a senior research analyst at the MedStar Health National Center for Human Factors in Healthcare.
Raj M. Ratwani is the director of the MedStar Health National Center for Human Factors in Healthcare, vice president of scientific affairs at the MedStar Health Research Institute, and an associate professor at Georgetown University School of Medicine.
Katharine T. Adams is a data scientist at the MedStar Center for Biostatistics, Informatics, and Data Science.
Rebecca Jones (rebejones@pa.gov) is director of Data Science & Research at the Patient Safety Authority.
Seth Krevat is the senior medical director at the MedStar Health National Center for Human Factors in Healthcare and an assistant professor at Georgetown University School of Medicine.
PA-PSRS is a secure, web-based system through which Pennsylvania hospitals, ambulatory surgical facilities, abortion facilities, and birthing centers submit reports of patient safety–related incidents and serious events in accordance with mandatory reporting laws outlined in the Medical Care Availability and Reduction of Error (MCARE) Act (Act 13 of 2002).23 All reports submitted through PA-PSRS are confidential and no information about individual facilities or providers is made public.
.jpeg)



.jpeg)