Introduction
Surgical specimen handling is a multistep process that includes ordering, collecting, labeling, preserving, transporting, testing, and reporting results so the patient receives a diagnosis or treatment plan. The individual steps of the specimen-handling process are categorized into phases, including the preanalytic phase (starting with the decision to collect a specimen until the specimen leaves the operating room), analytic phase (starting at the time the lab personnel receive the specimen until the testing is completed), and the postanalytic phase (starting when the testing is completed and lasting until the results are reported).1,2 Multidisciplinary collaboration is required between the surgeon, circulating nurse, surgical technologist, and pathology team to process a surgical specimen successfully. The specimen-handling process is an inherently complex workflow and problem-prone. When surgical specimens are mishandled, it contributes to increased morbidity, mortality, and healthcare costs.1,2
Problem Description
This project took place at a healthcare network of nine hospitals and four ambulatory surgery centers in the northeast United States. Approximately 22,500 specimens are processed quarterly in the healthcare network, with an error rate of 1.6 per 1,000 cases. The network quality team identified specimen handling as a problem-prone workflow when two serious events prevented a patient from receiving a diagnosis or treatment plan. The National Quality Forum defines a serious event as a harmful clinical event that is largely preventable.3 One serious event involved a lost specimen, and one serious event involved a mislabeled specimen. The quality team conducted a root cause analysis (RCA) in collaboration with hospital leadership and identified opportunities to strengthen system procedures to prevent future errors. A recommendation of the RCA was to conduct a failure mode and effects analysis (FMEA) for the specimen-handling workflow to prevent future errors from occurring.
Specific Aims
The objectives of the project were to
-
Conduct an FMEA analysis of the specimen-handling process to identify and select high-risk workflows for a quality improvement project.
-
Design and implement evidence-based changes to improve specimen-handling workflows and prevent future errors with frontline staff input.
-
Monitor outcomes of the process improvement project and adjust as indicated to maintain success with the practice changes.
Available Knowledge
Surgical specimen errors are reported most often during the preanalytic phase, with rates of 45%–71% across all specimen-handling error reports.2 Common specimen-handling errors ascribed to the preanalytic phase include mislabeled or unlabeled specimens, mismatches between specimens and the requisition form, incorrect order entry, lost specimens, incorrect preservation methods, transportation delays, or delivery to the incorrect lab location.1 Factors that contribute to specimen-handling errors include workflow variations and workarounds for collecting and processing specimens; knowledge and competence deficits; incomplete policies and procedures; failure to follow policies and procedures; environmental factors, including distractions or interruptions; miscommunication; and human factors for errors.1
Reason’s Swiss cheese model of system accidents is commonly used to explain the impact of human factors on errors in complex systems like healthcare. The Swiss cheese model describes the interplay between errors, system failures, and safe practices.4 Errors are defined as unintentional deviations from safe practices and are classified into two categories, active and latent.4 Active errors are events that occur at the point of contact between frontline personnel and a system interface and include errors of planning (mistakes) and errors of execution (slips or lapses).4,5 Contributing factors for mistakes include knowledge, skill gaps, lack of training, or narrowed focus.4 Contributing factors for slips or lapses include fatigue, noise, distractions, performance pressure, and inability to recall information to complete the task.4 Latent errors describe system design flaws like institutional factors, work environment factors, or team factors contributing to active errors.4,5
High-reliability organizations focus on eliminating errors by introducing practices that make the system error-resistant.6 Systems-focused error prevention strategies include cognitive aids, reporting systems, communication techniques, and effective training.6 Cognitive aids are just-in-time resources like algorithms or checklists that provide memory or decision support to frontline staff so they can correctly complete protocols or tasks. Successful cognitive aids are based on policies or guidelines, provide relevant information while eliminating unnecessary information, and organize information in the correct sequence.6 Hospitals use reporting systems to identify near misses and actual events, complete analysis of contributing factors, and strengthen systems to prevent future errors.6 Accurate and timely communication is critical to a safety culture.
The healthcare industry has adopted closed-loop communication techniques and structured communication templates from aviation and military training through programs like TeamSTEPPS and crew resource management.7,8 Training and competency evaluation are conducted at predetermined and periodic intervals to ensure that frontline staff have the knowledge, skills, and behaviors to successfully perform work tasks and support the safety culture within the organization.6 Finally, there are multiple tools to analyze errors and prevent future occurrences using a systems approach, including the RCA and FMEA. The RCA is a retrospective method used to analyze contributing factors and identify actions to implement to prevent a future occurrence.5 In contrast, the FMEA is a prospective method to identify problem-prone steps within a complex process to design and implement systems changes that would prevent future errors.9
The 2023 Association of periOperative Registered Nurses (AORN) Guidelines for Perioperative Practice addresses 21 topics in the section on specimen handling. Topics pertinent to this project include intraoperative team communication, transfer from the sterile field, handling, containment, labeling and requisition forms, policies and procedures, education, and quality. First, team communication should start at the preoperative briefing when the surgeon identifies specimen collection needs. AORN advocates for using a read-back method during any handover process and verbal confirmation of the correct specimen, labeling, number of specimens, requisition, and preservation methods.10 Next, the specimen will be transferred as soon as possible from the sterile field, and the specimen will be contained and labeled immediately, labeling one specimen at a time, and the specimen identification will be confirmed verbally.10 Finally, ensure multidisciplinary involvement in the development and review of policies and procedures, assess staff knowledge and competency levels, monitor and evaluate data on specimen handling, implement changes in specimen management based on data, and use a systems approach to reduce the risk of specimen-handling errors.10
Methods
The project team selected the Centers for Medicare & Medicaid Services (CMS)–supplied FMEA tool Guidance for Performing Failure Mode and Effects Analysis With Performance Improvement Projects as the framework for this project.9 This tool identifies seven key steps to complete an FMEA, described in this section as it applies to this project. The project was initiated in September 2022 by identifying key stakeholders to participate in an interdisciplinary committee to complete the FMEA process. In October 2022, the project leaders conducted a kickoff meeting to state the project objectives and desired outcomes and evaluate best practices for specimen handling. The project team mapped the process flow identifying each step of the specimen-handling process between November 2022 and January 2023. The identification and scoring of failure modes was completed by March 2023. The project teams created action plans for the top 10 highest risk failure modes between April 2023 and July 2023, with network socialization of the project plan in August 2023. Auditing and follow-up of strategies began in September 2023. Sustainability continues to be evaluated.
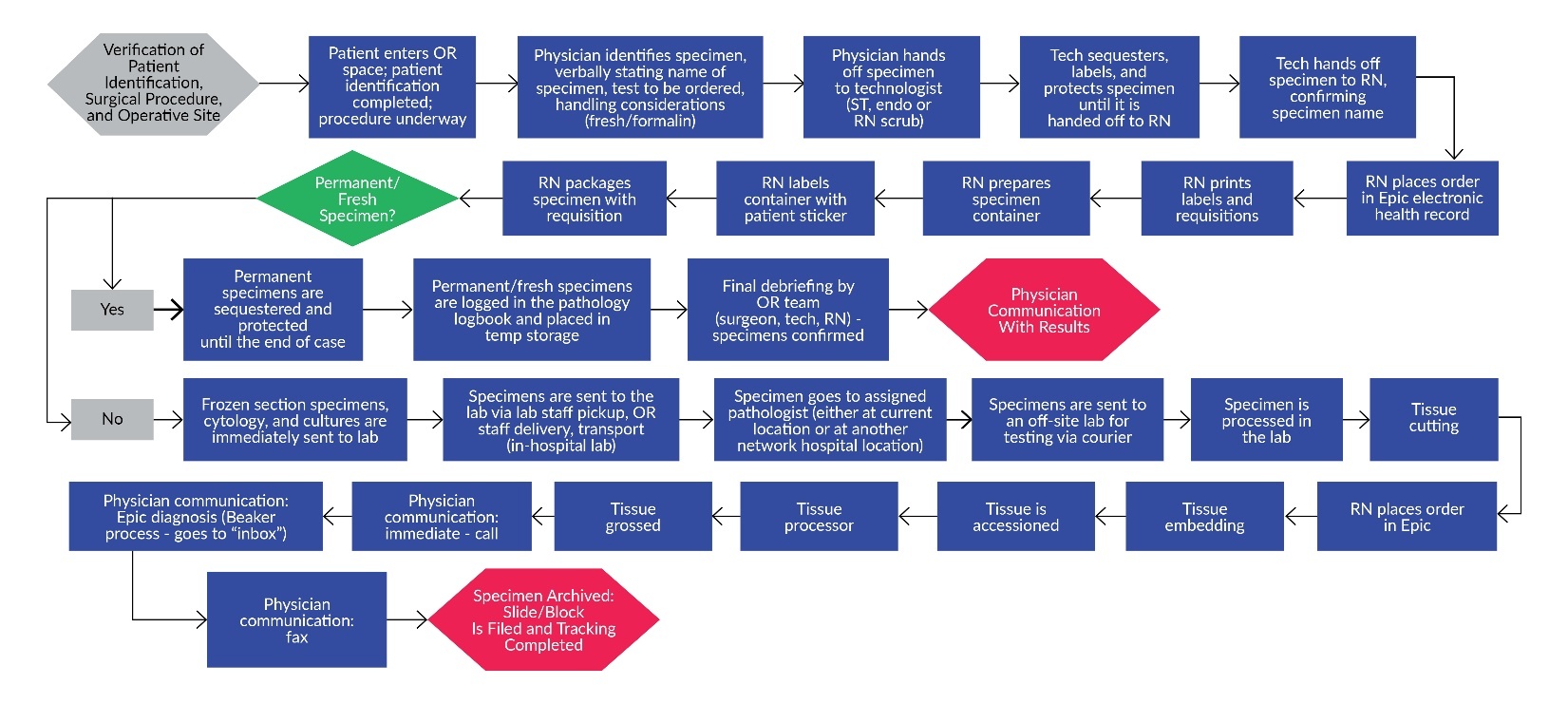
The first step of FMEA is to select a topic. The scope of this FMEA was specimen handling from operating room (OR) to result completion. The process steps include preoperative huddle communication to identify potential specimens, verbal specimen orders, order entry, specimen retrieval, specimen hand-off, specimen preparation and labeling, specimen transport, specimen testing, and specimen results. The opportunity for error and patient impact were key drivers for choosing this high-risk clinical process.
The second step is to create a project charter and identify the key stakeholders. The project charter described the project’s scope, identified project participants, and set objectives and outcomes. Three key experts led the project in specimen handling and performance improvement. The facilitators included the network director for Quality and Patient Safety to guide the team with the FMEA methodology, the director of Nursing Education and Professional Practice: Perioperative, Procedural, and Paraprofessional Education, and the quality director for the Pathology Institute. Additional team members included one surgeon, one pathologist, one OR director, two OR managers, one OR nurse, one laboratory manager, and hospital-specific patient safety officers. Each committee member was considered a subject matter expert (SME) and voluntarily committed to participate in the FMEA process. To complete the FMEA process, the team met every other week.
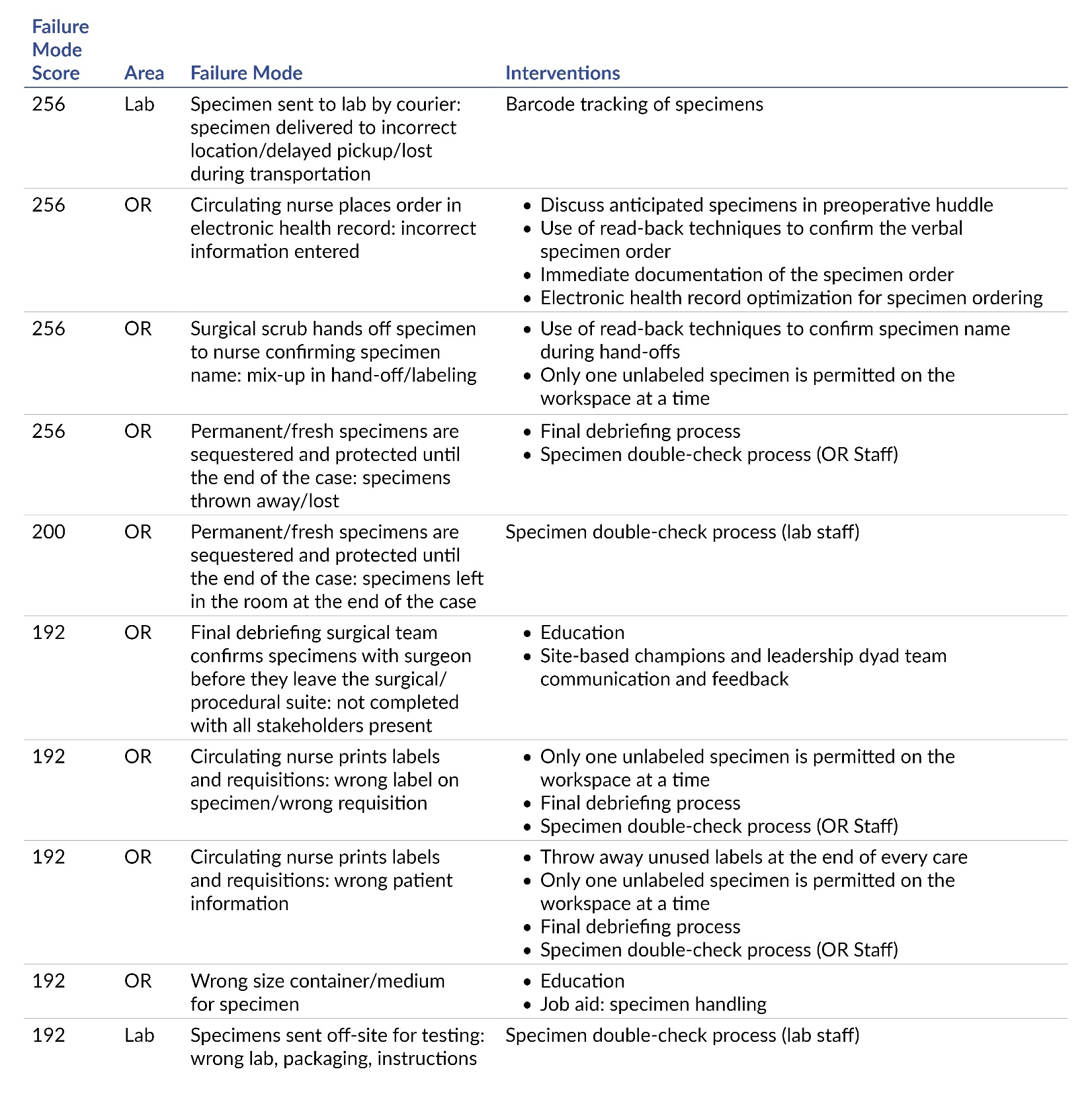
The third step of FMEA is to describe the process. A process flow diagram was completed, identifying each step of obtaining a specimen from the OR through result completion (Figure 1). In the fourth step, the project team identifies what can go wrong during each process step. The SMEs participated in a series of brainstorming sessions that identified contributors to error, or failure modes, throughout each step in the specimen-handling process. The facilitators identified the failure modes by adding each failure mode to the FMEA tool (Figure 2).
In the fifth step, the project team selects problems to work on. Eighty-two failure modes were identified in the brainstorming sessions. The SMEs evaluated each mode on a scale from 1–10 (10 was the highest concern) for likelihood to occur, likelihood for detection, and level of severity. A risk profile number was calculated, and the top 10 failure modes were identified by the facilitators for action planning (Table 1). The top 10 list was socialized with the FMEA team and was adopted as the key areas of concentration for improvement activity.
Interventions
In the sixth step of FMEA, the project team designs and implements changes to reduce problems. The facilitators worked with their SME and departmental teams to develop action plans for each of the top 10 failure modes identified during the FMEA process. Specific activities were shared with the entire FMEA committee and approved for implementation.
The OR team created a network specimen-handling council comprised of nurses and surgical technologists representing the hospital and surgery center OR and gastrointestinal (GI) lab departments. The council created a network-standardized, specimen-handling procedure based on the 2023 AORN guidelines. Key interventions selected that support accurate specimen-handling workflows included:
-
Discussion of specimens during the preoperative huddle
-
Immediate documentation of the specimen orders by the nurse as the physician is retrieving the specimen
-
Use of read-back methods to confirm the specimen name during every hand-off
-
Implementation of a double-check process by the surgical scrub and circulating nurse to verify all specimens before they leave the procedural room for specimen transport
-
Implementation of a double-check process by the laboratory to verify all specimens at the time of pickup in the procedural area, education, and widespread socialization of the project10
Each OR and GI lab manager selected a physician champion, and the project was presented at each facility’s perioperative executive meeting. An education module was assigned to all OR nurses, surgical technologists, and endoscopy technicians, and huddles were used to reinforce the new process.
The lab team focused on standardizing a log utilized to document specimens and a process to barcode specimens to track delivery. A redesign of the specimen log sheet both in the OR space and for dropped-off specimens allowed for better identification of labeling and ordering errors. Lab staff were instructed not to fix errors but to ask the submitting department to correct issues before accepting specimens. This led to the identification of information technology solutions for ordering difficulty. In addition, for specimens collected in the ambulatory space or remote surgical centers, the team set up weekly meetings with our contracted courier service to implement barcoding for tracking purposes. A portal system was designed for clients to place pickup orders and allowed for printing labels with the correct address and barcodes. Couriers were instructed to scan the specimen barcode at its origin and final lab destination. A weekly report of scanning activity was reviewed at each meeting, and improvement opportunities were identified.
In the seventh FMEA step, the team measures the success of process changes. The facilitators worked with the electronic incident reporting administrator and patient safety officers to develop a specific report related to specimen handling for each location in the network. This report was received monthly and shared with the FMEA SME and the hospital-based patient safety committees (PSC). Additionally, the OR teams developed an audit of the specimen process to confirm that the specimens were correctly labeled, and both double-check verifications were completed. The OR audit results are reported at the facility’s PSC meetings. Fifteen random audits were conducted in the OR until the department had three consecutive months with equal to or greater than 85% compliance with each process using a standardized form. Finally, the facilitators sustained the project by reviewing and reporting specimen-handling errors monthly. The facilitators will initiate a quality improvement cycle if the number of specimen errors exceeds the baseline recording or if the type of errors reported is serious or problematic.
Measures
Process measures and outcome measures were recorded to determine compliance with the project and impact on outcomes. The process measures for the project included an internally created audit to determine compliance with the new specimen-handling process in the OR, for lab pickup, and for barcode scanning with the courier service. Outcome measures include the number and type of reported mishandled specimens obtained from the network patient safety reporting system. Process and outcome measures were submitted to the network specimen-handling committee. The number and type of reported mishandled specimens by month and the results of the monthly OR audit were reported monthly to the project team, the network specimen-handling committee, and the perioperative leaders. The perioperative leaders report this information quarterly at their perioperative executive committee meetings and the facility patient safety meetings.
Results
Using the FMEA process, the SMEs identified 82 failure modes throughout the 28 steps in specimen handling. Once the top 10 failure modes were identified and process improvement activities commenced, workflow enhancements were monitored with the network patient safety reporting system, monthly OR audits, and partnering with the contracted courier service to track and resolve specimen transport issues. Monthly OR audits found that 16 out of 23 departments demonstrated three consecutive months of greater than 85% compliance with new workflows during the project’s first three months. Twenty of the 23 departments demonstrated three consecutive months of greater than 85% compliance with new workflows within six months.
Weekly meetings with the contracted courier service to create a transport issue tracking log demonstrated improved specimen tracking with newly implemented package labels and barcodes. Barcoding of packages and standardizing labels for packages was effective in the timely delivery of specimens. The team did not report any lost specimens; there was an improved delivery to the correct lab for testing. At the time of publication, the barcode scanning compliance was 83% with pickup and delivery. Reported near miss and actual mishandled specimens increased by 26% in the first three months after implementation when compared with the calendar year 2023/24 baseline (Figure 3). However, there were zero reports of serious events post-project implementation.
Discussion
Consistent with the literature, the project leaders found inconsistencies in workflows, workarounds, human factors for error, and knowledge deficits during the FMEA process.1 The project team used the FMEA methodology to pinpoint areas of opportunity and prioritize strategies based on potential impact. There were crucial learnings throughout the process. The team noted the importance of defining reporting criteria and emphasizing the process for submitting actual and near miss specimen-handling errors. Project leads determined it was critical to collaborate with stakeholders, including the contracted courier service and the transporters responsible for specimen pickup and delivery. The involvement of the operating room frontline staff was key to identifying gaps in the process during the FMEA review. Human factors for error identified during the FMEA include distractions, miscommunication, knowledge gaps, and competing priorities. In addition, creating a standard specimen-handling process was pivotal to improving communication between staff and providers. Focusing on improvement efforts throughout the continuum positively influenced willingness to participate in the review process.
Post-intervention implementation, there was an increase in events reported in the network patient safety reporting system from the baseline data time frame, signifying a heightened awareness of the process improvement initiative. The increase in reporting validated the engagement of staff related to the specimen error prevention project. Additionally, the increased reporting of actual and near miss specimen-handling errors assisted the team with evaluating whether the action items implemented sufficiently addressed the opportunities identified in the FMEA. This is similar to findings by Simeile et al. suggesting that the use of cognitive aids, effective reporting systems, closed-loop communication techniques, and effective education are key drivers in the success of eliminating errors in high-reliability organizations.6
Multiple factors facilitated change in this project, including frontline staff engagement and empowerment in designing the specimen-handling procedure and evidence-based process changes, leadership support, and project reporting in multiple avenues. Perioperative nurses and technologists reviewed the literature and AORN guidelines to recommend practice changes, creating ownership of the project and positive influence with their peers. Leadership at all levels was aware of the project through communication channels and presentations, creating high support and visibility. The OR and GI managers partnered with physician champions, increasing buy-in and support for the evidence-based practice changes. Finally, frequent reporting through multiple meetings and communication channels created space for interprofessional collaboration and problem-solving, advancing the project’s success.
Conclusions
The project team conducted a quality improvement project using the FMEA tool because the evidence-based methodology is used to evaluate complex processes that could impact patient safety. The project team identified 82 failure modes that could occur during specimen handling in the OR and GI lab. The top 10 failure modes were selected based on risk score and evidence-based interventions were designed to mitigate patient harm. Over six months, process and outcome measures were tracked, which revealed compliance with industry guidelines and the new network procedures for 20 out of the 23 impacted departments. This project engaged frontline staff in problem-solving and process improvement, which was key to the success of the program. The project has been sustained through monitoring and reporting of the process and outcomes measures over 11 months with no serious events reported. The approach was logical and detail-oriented, enabling a thorough review of the problem and identifying action items to prevent harm.
Data Availability Statement
The raw/processed data required to reproduce the above findings cannot be shared at this time due to legal/ethical reasons.
Disclosure
The authors declare that they have no relevant or material financial interests.
The organization’s Human Research Protection Office guidance and procedure noted that data-guided activities designed to implement promising ways to improve clinical care, patient safety, and healthcare operations were exempt from Institutional Review Board approval. The activity was designed to bring immediate positive changes in healthcare delivery programs in the local setting. The intent was limited to improving care and operations. There were no patient identifiers for this quality improvement project.
About the Authors
Sara Angelilli (sara.angelilli@ahn.org) currently serves as the director of Clinical Nursing Operations at Allegheny Health Network, providing strategic and operations support to nine hospitals. Angelilli received her doctoral degree with a specialization in nursing administration and dual master’s degrees in nursing education and leadership and industrial and organizational psychology. She is dual certified in perioperative nursing and nursing professional development.
Lisa Feathers is the director of Quality in the Pathology Institute of Allegheny Health Network in Pittsburgh, Pennsylvania. She is a passionate advocate for quality and patient safety in laboratory medicine, with over 30 years of experience in the field. Feathers holds a Bachelor of Science degree in medical technology from West Virginia University (1987) and a certificate in process improvement from Intermountain Health system (2010).
Michelle McGonigal is a healthcare leader dedicated to optimizing patient outcomes and fostering a culture of excellence. As network director of Quality and Patient Safety at Allegheny Health Network, she ensures the highest standards of care. Dr. McGonigal’s expertise spans nursing, quality improvement, and patient safety, and she holds multiple certifications. She earned her doctor of nursing practice degree from Waynesburg University. She is a sought-after speaker and author, sharing her insights on quality and patient safety. Dr. McGonigal’s leadership style emphasizes collaboration, empowerment, and patient-centered care, creating high-functioning teams committed to continuous improvement.




_vs_post-intervention_(nov._202.jpeg)


_vs_post-intervention_(nov._202.jpeg)