Introduction
Medical equipment, supplies, and devices (ESD) are used in nearly all healthcare environments to diagnose and treat patients and are instrumental to the care process. More than 2 million different kinds of medical devices are available worldwide.1 Depending on the type of ESD, there may be different standards and/or requirements for effectiveness, reliability, and safety.2–4 There are also federal oversight organizations, such as the Food and Drug Administration (FDA), that provide regulatory frameworks and may require reporting of ESD issues that impact patient safety.5 Despite these requirements and oversight, ESD-associated safety issues occur and can result in patient harm.6–9
Quantifying the frequency of ESD-related safety issues across healthcare settings has been difficult.10,11 However, numerous studies have shown the impact of ESD issues on patient safety in specific contexts.6–9,12,13 For example, a systematic review of surgical technology found that failure of equipment and technology account for a median of 23.5% of all errors, with a median of 0.9 equipment errors per surgery.12 A United Kingdom–based study analyzing patient safety incidents from intensive care units found that nearly 8.5% of those reports were associated with equipment-related issues.6 One study sought to estimate medical device–associated events from emergency department visits and suggests that regulatory surveillance systems grossly underestimate the number of actual events by as much as four times.13
Several recommendations have been proposed to address ESD-related safety issues. There have been requests for oversight agencies to improve their policies and regulations by leveraging advancements in regulatory science.11,14 There have also been calls for healthcare facilities to improve surveillance of medical device–related issues.11,15 Some medical specialties have suggested that use of and improvements to registries to track ESD use would support better identification of safety issues.10,16 These recommendations may all have an impact on improving ESD safety; however, they are difficult for a single healthcare facility to implement on its own.
In this study, we analyzed a subset of ESD-related patient safety event reports to identify the type of safety issue described and the specific ESDs and components or ESD subtypes associated with the safety issue. There are numerous taxonomies to describe safety issues associated with ESDs. Some taxonomies focus on distinguishing between user errors, defined as instances in which the user incorrectly interacts with the ESD, and malfunctions, defined as instances in which the ESD does not function as intended by the manufacturer.6–8,17,18 To better understand the nature of ESD-related safety issues, our analysis utilizes a human factors approach that focuses on how a user interacts with ESDs to complete their work tasks. With this approach, ESD-related safety issues can be characterized as malfunctions, which are ESD failures that may prevent the user from using the ESD; ESDs with missing parts; ESDs not being sterile, and design-related issues that impact how the user interacts with the ESD (i.e., usability issues). These four categories leverage aspects of the FDA’s Manufacturer and User Facility Device Experience (MAUDE) database taxonomy.19 Based on this analysis, we developed a patient safety procurement assessment tool to guide healthcare facilities in their selection process. Healthcare facilities may be able to improve their procurement processes to make certain ESD-related safety issues less prevalent and mitigate the risks associated with these issues.
Methods
Data Source
We analyzed patient safety event reports submitted to the Pennsylvania Patient Safety Reporting System (PA-PSRS)[1] between January 1, 2019, and December 31, 2021. All nonfederal, acute care facilities in Pennsylvania are required to report patient safety events through the PA-PSRS system. Each report contains a single event type category and free-text description of the safety issue, along with responses to many additional structured and unstructured questions. Our analysis focused on the Equipment, Supplies, and Devices event type category, as assigned by the reporter, which consisted of 24,660 reports from 334 facilities.
Topic Modeling Sampling Strategy
To understand the breadth of ESD-related safety issues, and the specific ESDs and components or ESD subtypes involved, we used a topic modeling approach to identify reports for manual review. This approach enables rapid identification of reports that have similar information, such as similar types of ESDs, from a large database of reports. We then manually reviewed a selection of reports from each topic (described below). This approach enables a broader understanding of safety issues impacting a variety of ESDs compared to a random sampling of reports, which would be skewed toward ESDs that are reported more often. To identify these topics, we applied a technique called latent Dirichlet allocation (LDA) modeling, commonly called topic modeling, to the event description of each ESD report.20,21 This technique uses statistical probabilities to create sets of words that are more likely to represent a topic or group and provides the probability of each free-text report being associated with each topic group given the words in the report. Topic modeling requires some preprocessing of event description text as well as model development iterations to identify the ideal number of groups of related ESDs. This topic modeling approach led to 10 groups of related ESDs. Upon clinical review, it was determined that one topic group was not relevant to the scope of this work, and thus, nine groups were included in the analysis.
Coding Process and Analysis Methods
To understand the types of safety issues and other characteristics associated with reports under each topic of related ESDs, we reviewed the 50 most relevant reports per topic based on the highest coherence scores. Each report was manually coded by a human factors expert and a physician with safety expertise to identify the ESD-related safety issue (e.g., malfunction, usability); the ESD(s) associated with the report (e.g., intravenous [IV], ventilator, patient bed); and the associated component(s) (e.g., battery, tubing, screen, button) or ESD subtype(s) (e.g., X-ray, CT). A component was defined as a specific part of an ESD; for example, a wheel lock is a component of a bed. An ESD subtype was defined as a specific ESD that is part of a broader class of ESDs; for example, an X-ray is a subtype of imaging ESDs. The ESD-related safety issues, definitions, and examples are shown in Table 1 and are based on the FDA’s MAUDE taxonomy. One ESD-related safety issue was coded for each report; when multiple safety issues were described, only the initiating event was coded. If a report did not describe an ESD-related safety issue or had insufficient information to determine whether it was ESD-related, the report was excluded from our analysis and replaced with the report with the next highest coherence score from that topic. For each report, the ESD type and component or ESD subtype were noted if they were explicitly mentioned in the event description, with multiple ESDs and components or subtypes coded if referenced in the report.
After the coding was complete, a descriptive analysis was performed to identify patterns in the coded data. The reports comprising these patterns were reviewed by the two subject matter experts to identify clinically meaningful insights. These insights are described in the results following the descriptive analyses.
In addition to coding of the free-text description of each report, one structured category was analyzed: Care Area Group, which indicates the broader care area group associated with the reported patient safety event based on the care area type assigned by the reporting facility.
Results
ESD-Related Safety Issues Identified in Reports
Across all ESD reports reviewed, malfunction was the most frequent ESD-related safety issue coded (n=365 of 450, 81.1%). Among the coded malfunctions, software/output problem (n=122 of 365, 33.4%) and general malfunction (n=103 of 365, 28.2%) were most frequent. The prevalence of software/output problems speaks to the increasingly large role technology plays in the delivery of healthcare and the large number of general malfunctions is indicative of the lack of information found in the free text of patient safety event reports. Other malfunctions included material integrity (n=72 of 365, 19.7%) and activation, positioning, or separation (n=68 of 365, 18.6%). A common theme in the activation, positioning, or separation malfunction category was failed insertion and removal of surgical instruments by the operator and trigger failures for surgical sealant or closure devices. Sterilization (n=40 of 450, 8.9%), usability (n=36 of 450, 8.0%), and physically missing (n=9 of 450, 2.0%) accounted for the remainder of the coded reports. Many of the sterilization and physically missing reports were related to preoperative logistics involving instrument preparation. A review of the reports associated with sterilization and physically missing issues were both related to types of process errors, with a prevalence of reports indicating issues with material handling and preparation by members of the healthcare team.
ESD, Component/Subtype, and Most Frequent ESD-Component/Subtype Pairings
At least one ESD was identified in each of the 450 reviewed reports, with some reports describing multiple ESDs, resulting in 64 unique ESDs identified and a total of 462 ESDs coded across all reports. To gain an understanding of the most frequently mentioned ESDs, Table 2 shows those ESDs that appeared 1% or more of the time. In total, these 17 ESDs were coded 386 times, and the five most frequent were infusion pump (n=50 of 462, 10.8%), instrument set (n=49, 10.6%), IV (n=49, 10.6%), imaging equipment (n=45, 9.7%), and ventilator (n=43, 9.3%). Two of the three most frequently reported ESDs, infusion pump and IV, were related to the delivery of medications other than via the enteral route.
At least one component/subtype was identified in 348 (77.3%) of the 450 reports reviewed, with some reports describing multiple components/subtypes, resulting in 97 unique components/subtypes identified and a total of 464 times that a component/subtype was coded across all reports reviewed. To gain an understanding of the most frequently mentioned components/subtypes, Table 3 shows those that appeared in three or more reports. In total, these 49 components/subtypes were coded 405 times and the most frequent were alarm/alert (n=45 of 464, 9.7%), tubing (n=31, 6.7%), tray (n=28, 6.0%), telemetry-related (n=24, 5.2%), and tip (n=24, 5.2%). It should be noted that alarm/alert, balloon, foley, laser, light cord, needle, scissors, screwdriver, pulse oximeter, table and wire are listed as both ESDs and components/subtypes, as sometimes these were the primary ESD noted in the report narrative and sometimes these were described as a component/subtype of an ESD.
To gain a better understanding of the components/subtypes associated with each ESD we examined ESD-component/subtype pairings. To do this, we looked at the ESDs that were reported 3% or more of the time, which resulted in the eight most frequently reported ESDs. Under each ESD we then looked at the components/subtypes with a frequency of 3 or more per ESD and the results are displayed in Table 4. The gray-shaded rows show the ESDs coded, and the total number of components/subtypes associated with that specific ESD. For each component/subtype under each ESD, the frequency count and percentage relative to the total number of components/subtypes per ESD are provided (e.g., 22.2% of the total components/subtypes associated with infusion pumps are alarms/alerts). Two components/subtypes (alarm/alert and display) were found to be dominant across multiple ESDs. Alarms/alerts appeared as a top ESD-component/subtype pairing for phones (n=11 of 25, 44.0%), ventilators (n=17 of 52, 32.7%), infusion pumps (n=12 of 54, 22.2%), and patient monitors (n=8 of 51, 15.7%). Displays were also found to be a top ESD-component/subtype pairing across ESDs for ventilators (n=8 of 52, 15.4%) and patient monitors (n=6 of 51, 11.8%). For instrument sets, the components/subtypes identified most frequently were tray (n=28 of 65, 43.1%) and wrapper (n=14 of 65, 21.5%), and all point to these components/subtypes being problematic in the sterilization of equipment and instrument sets.
ESD-Related Safety Issues by ESD
Table 5 shows the frequency count and percentage of safety issues associated with ESDs that were mentioned 1% or more of the time (17 unique ESDs mentioned 386 times). Software/output malfunctions were dominant with infusion pumps (n=43 of 123, 35.0%), imaging equipment (n=24 of 123, 19.5%), and patient monitors (n=24 of 123, 19.5%). While material integrity malfunctions were the least common malfunction, these were prevalent with IVs (n=17 of 39, 43.6%) and needles (n=9 of 39, 23.1%). Activation, positioning, or separation malfunctions were associated with a variety of ESDs compared to other safety issues. Outside of malfunctions, notable results also included usability issues associated with patient monitors (n=9 of 28, 32.1%), instrument sets (n=7 of 28, 25.0%), and patient beds (n=5 of 28, 17.9%), and sterilization issues associated with instrument sets (n=37 of 38, 97.4%).
Care Area Group by ESD
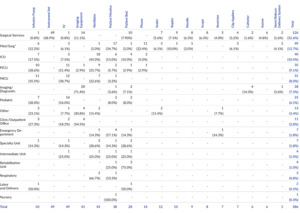
Table 6 shows the frequency count and percentages of reports for care area group across the 17 most frequently reported ESDs. Surgical services had the largest number of reports across care area groups (n=126 of 386, 32.6%), followed by med/surg (n=49 of 386, 12.7%), intensive care unit (ICU) (n=40 of 386, 10.4%), pediatric intensive care unit (PICU) (n=35 of 386, 9.1%), and neonatal intensive care unit (NICU) (n=31 of 386, 8.0%). The surgical services care area group had the greatest breadth of associated ESDs, with instrument sets (n=49 of 126, 38.9%), imaging equipment (n=14 of 126, 11.1%), and patient beds (n=10 of 126, 7.9%) as the three most prevalent ESDs. Med/surg also had several associated ESDs, with patient monitor (n=17 of 49, 34.7%), phone (n=11 of 49, 22.4%), and infusion pump (n=6, 12.2%) as the three most prevalent ESDs. Another notable finding includes the concentration of reports in the PICU, NICU, and pediatric care area groups, totaling 23.6% (n=91 of 386) for care area groups associated with pediatric populations. The PICU, NICU, and pediatric care area groups were primarily related to IVs (n=37 of 91, 40.7%), infusion pumps (n=28 of 91, 30.8%), and ventilators (n=16 of 91, 17.6%).
Discussion
The results highlight pervasive contributions of ESD malfunctions to patient safety risks. Software/output problems were found to be the dominant malfunction, primarily associated with infusion pumps, patient monitors, and imaging. General malfunctions were the second highest malfunction, followed by material integrity and activation, positioning, or separation. Sterilization comprised nearly 10% of the ESD-related safety issues. Looking at the component/subtypes associated with the ESD reports, alarm/alert was the most frequent and was often identified with infusion pumps, ventilators, patient monitors, and phones. Further research is needed to identify how alarm/alert issues may be contributing to ESD patient safety risks. Usability was the second least frequently coded ESD-related safety issue across all reports. These descriptive analyses and qualitative insights can inform ESD patient safety practices.
Addressing Malfunctions
The prevalence of software/output malfunctions suggests a need to better understand how healthcare providers are interacting with different ESDs and components/subtypes to ensure safe use. There are opportunities to address software/output issues and mitigate safety hazards before they result in patient harm. First, these ESDs should be rigorously assessed during procurement to identify potential issues before purchase. This will prevent ESDs that may pose safety challenges from being introduced in the care environment. A patient safety procurement assessment tool, described later in the discussion, can support healthcare facilities in this process. Second, healthcare facilities should monitor ESDs for malfunctions with biomedical engineers and the ESD manufacturers to understand the context in which these malfunctions are occurring and determine how to best address these safety issues.
Some of the ESD malfunctions were coded as general malfunctions in part because not enough information was provided to identify a more specific code. Healthcare facilities should ensure detailed information is being collected and reported so that the malfunctions can be better understood. For example, certain reports were associated with broken objects during surgery or procedures, specifically needles, wire, balloons, and catheters. Of these broken components/subtypes, some were intentionally retained in the patient due to a high risk of removal and others were unable to be found. As broken components can lead to safety risks or patient harm, manufacturers could explore needle, wire, balloon, and catheter design and reliability to identify possible sources of defects and material strength issues. In addition, when objects break, these safety issues should be investigated to determine what instruments were used to grasp and present them to the surgeon along with the user’s understanding of how to use these tools.
A review of reports coded in the activation, positioning, or separation malfunction category revealed that a common theme was failed insertion and removal of surgical instruments by the operator. Further investigation is needed to determine if insertion and removal processes can be improved through more user-centered design modifications or training enhancements. In addition, trigger failures for surgical sealant or closure devices were a dominant theme. This trigger failure theme suggests that the frequency and comprehensiveness of preventive maintenance audits associated with the relevant ESDs (e.g., stapler, sealer) should be reviewed to determine if adjustments are needed for manually activated tools. Optimizing preventive maintenance is critical as a risk mitigation measure but must be balanced against site resources and frequency of safety event occurrence for the specific ESD.22
Sterilization and Physically Missing Issues: Process Management
While reports coded as physically missing were not necessarily indicating safety issues related to ESDs themselves, but the processes around handling them, it was a relevant finding worth noting. Specifically, the review showed dominant themes related to preoperative logistics involving instrument preparation (nonsterile instruments found or instruments missing in trays). This information highlights the need for process improvement initiatives to understand the potential causes of these events. Surgical equipment checklists with explicit reference to equipment availability and sterility have been recommended in these contexts to provide an additional preventive mechanism.23
Prominence of Usability Issues
Although usability was not a prominent patient safety issue in the patient safety event reports we reviewed, usability issues have been shown to be directly associated with patient harm in research focused on medical devices.24,25 One reason for this may be the difficulty for reporters to identify and describe usability-related issues. There is a need for a better way to capture usability- and safety-related issues with medical devices.
The most commonly coded ESDs among usability-related safety issues were patient monitor, instrument set, and patient bed. Of clinical significance, a review of patient bed reports often described bed position controls. Bed issues were also prominent among the malfunction categories (general; material integrity; activation, positioning, or separation), due to issues with position changes of the bed and patient transfer into the bed. Even though bed malfunctions were largely associated with manual handling and human-system interaction, due to sparse narrative detail, it was unclear if usability issues influenced the outcome.
Care Area Group Safety Issues
A concentration of reports in PICU, NICU, and pediatric care areas was found for IVs and infusion pumps. This research highlights the need to further explore why pediatric and neonatal care areas are experiencing a large percentage of safety issues related to the tools associated with the infusion of medications. ESD-related safety issues associated with patient monitors and phones were found to be prominent within the med/surg care area group in comparison to the ICU, PICU, and NICU. These results may be indicative of the increased staffing in intensive care units likely leading to fewer monitoring misses and easier communication, with more reliance on monitoring software and technology for communication occurring in med/surg care areas.
Policy Implications
Our results have policy implications for federal organizations like the FDA, as well as for state-level agencies and other stakeholders. For high-risk ESDs that have clear patient safety consequences, guidelines for design and standards for malfunction rates may be warranted. There may be an opportunity to improve manufacturer communication to healthcare facility customers about known malfunctions, and guidance for remediation should be provided in a timely fashion. In addition, healthcare facilities may need certain standards in place to rigorously test ESDs for malfunctions to prevent malfunctions during critical patient care activities. Policies internal to healthcare facilities may need to include reliability audits for ESD preventive maintenance and enhanced training and procedural supports for high-risk processes involving ESDs. Further, in addition to the usability testing performed by many manufacturers, healthcare facilities should also perform internal testing for their specific user groups, as procedural needs may vary among users at different sites.
Patient Safety Procurement Assessment Tool
Improved ESD safety assessment during procurement could improve the likelihood of purchasing ESDs that are well designed with low malfunction rates. Preventing poor quality ESDs from being adopted by healthcare facilities is the most proactive patient safety approach a healthcare facility can take. Online Supplement Appendix A contains a patient safety procurement assessment tool that healthcare facilities can use to guide their vetting and selection of ESDs. While many healthcare facilities may already be using similar tools and may already follow the recommendations provided below, not all healthcare facilities have adopted these practices.
To address ESD usability issues, healthcare facilities can do the following during procurement:
-
Assess the usability and safety of ESDs. Recognizing healthcare facilities have limited resources to conduct assessments, facilities should focus on ESDs that are used frequently and may pose the greatest risk of harm. There are several methods that can be used to do this. Formal usability testing can be conducted, which involves identifying the typical user group of the ESD and having those users complete typical tasks while measuring time to complete the task, error rates, and satisfaction. This process can be expensive and requires usability knowledge to effectively create scenarios and measure efficiency, effectiveness, and satisfaction. Another approach is to complete a rapid heuristic evaluation. Online Supplement Appendix A contains a tool to provide knowledge and guidance on how to conduct a heuristic evaluation. This tool can be used to assess the usability of any ESD and does not require usability domain knowledge. Healthcare facilities can also ask ESD manufacturers for information on how the ESD was usability tested and ask for measures of usability. Some manufacturers may provide this information.
-
Learn from other organizations. Other healthcare facilities that are already using the ESD could be contacted to inquire about usability and safety issues.
To address malfunctions, healthcare facilities can do the following during procurement:
-
Search publicly available databases that contain reports about patient safety issues associated with ESDs. For example, the FDA’s MAUDE database contains reports on safety issues associated with medical devices.19 These databases can provide insights on the types of malfunctions, or other issues, that have been reported about the ESD under consideration.
-
Ask the ESD manufacturer for malfunction rates and whether any issues have been reported by users. For new equipment with no history of use, ask the manufacturer about internal testing results related to malfunctions and usability. Compare malfunction information across products under consideration to determine which ESDs would be best suited for your facility.
-
Consider contacting other facilities that have already adopted the ESD being considered and ask the facility about malfunctions and other issues they may have experienced.
Limitations
This study is limited to the reports submitted to PA-PSRS over two years so the results may not be generalizable or inclusive of all ESD issues. Despite mandatory reporting laws in Pennsylvania, events are self-reported and may not represent all ESD-related events from the reporting healthcare facilities. Additionally, the search strategy was limited to the ESD event type, and ESD-related safety issues may be present in reports submitted under different event types. Furthermore, our analysis was limited to the information provided in the patient safety event reports and we were not able to follow up with reporters, healthcare facilities, or manufacturers for additional information about ESDs. COVID-19 may have impacted the number and types of ESD issues reported. In addition, if certain ESDs were recalled or highlighted to have certain malfunctions, this information may prompt healthcare workers to report on these issues more frequently. The topic modeling technique required preprocessing of ESD event description text, which included the removal of high-frequency and low-frequency words. This may have resulted in some relevant ESD information being removed from reports and not being included in the topic modeling results.
Conclusion
The continued occurrence of ESD-related safety issues, especially malfunctions, highlights the need for healthcare stakeholders to create more proactive and coordinated risk mitigation efforts. Oversight agencies can provide more optimal guidelines and standards to inform manufacturer design and development and can identify better ways to encourage use of these guidelines and standards. Manufacturers can better identify, measure, and share malfunction types and rates. Healthcare facilities can improve patient safety assessments during the procurement process.
Note
This study was approved by the MedStar Health Research Institute institutional review board.
Disclosure
The authors declare that they have no relevant or material financial interests.