Introduction
The world’s population has grown in number and age over time. It is estimated that the world’s population will grow by another 0.5 billion persons within the next seven to eight years, and over the next 25 years average life expectancy will increase by 4.5 years.1 An aging population with more chronic diseases poses a challenge to the healthcare system, as these patients are expected to be admitted more often and for a longer duration. Consequently, the number of admitted patients may exceed the limited number of available hospital beds. Therefore, it is necessary to reconsider where else patients can be observed within the healthcare system. Alternatives such as preventing admission, admission at home, or earlier discharge should be considered. If acute admissions are to be avoided, a need exists for identification of patients at high risk of getting admitted and readmitted.
It is well described that patients with chronic diseases have a considerably higher risk of admission and readmission, e.g., patients with chronic obstructive pulmonary disease (COPD) have a 30-days readmission rate of 20%.2–4 However, predicting early deterioration is challenging. Previous studies have found that spot measurement of vital signs at home were insufficient for predicting acute exacerbation of COPD (AECOPD)5–7 but prediction improves by adding daily self-assessment and measurement of C-reactive protein.6 Another alternative is to admit patients in their own homes.
Patients admitted in their own homes report greater satisfaction8,9 and are more physically active, and mortality may be lower,10 but concerns have been raised regarding home admission and safety. In recent years “telemedicine” has been a priority of politicians due to its potential to relieve healthcare professionals, identify deterioration early, and initiate treatments before illness necessitates admission.11 Continuous monitoring by wearable vital sign sensors is one aspect of telemedicine. The sensors exist and have been validated in-hospital; however, researchers must now determine feasibility for out-of-hospital use.
The aim of this study was to document feasibility of continuous home monitoring, described as duration of valid data collection, and to examine frequency and duration of deviating vital signs during the first days after hospital discharge.
Methods
Participants
Patients were eligible if they were 18 years or older, were admitted with an acute medical disease based on the International Classification of Diseases, Tenth Revision (ICD-10) classification and noted in the patient’s record, and had a discharge disposition to home. The patients were recruited at Bispebjerg Hospital, Copenhagen, Denmark, in the period August 2021 to May 2022. Patients were excluded if they were allergic to plaster, plastic, or silicone; had an implanted pacemaker or implantable cardioverter-defibrillator (ICD) device; or if they were not able to open the front door for investigator visits. Demographic data and 30-days follow-up were collected from the electronic patient records. The study was approved by the Committees on Health Research Ethics in the Capital Region of Denmark (H-20009132) and registered at ClinicalTrials.gov (NCT05223504). All participants provided written informed consent prior to inclusion.
No formal power calculation was performed, as this was a pragmatic feasibility study intended to provide basis for further studies.
Monitoring
The following wireless monitoring sensors were used to record vital signs continuously:
-
A single lead electrocardiogram (ECG) patch (Isansys Lifecare, Oxfordshire, UK) placed on the chest. The chest patch measured heart rate (HR) and respiratory rate (RR).
-
Nonin WristOx 3150 (Nonin Medical inc., Minnesota, USA), a bracelet connected to a finger pulse oximeter measuring peripheral oxygen saturation (SpO2).
-
A&D ambulatory blood pressure (BP) sensor (A&D Medical, California, USA), a compact, noninvasive, oscillometric upper arm BP sensor.
BP was automatically measured every 30 minutes during daytime (7 a.m.–9:59 p.m.) and every 60 minutes at nighttime (10 p.m.–6:59 a.m.). Measurements were sent via Bluetooth to a gateway placed in the patient’s home, from where data were downloaded on a dedicated secure server. If patients were out of Bluetooth range, data were stored on the sensors and transferred when within range, except for the BP sensor that required Bluetooth connection to collect data. Data was processed on the server, where an algorithm removed noise and artifacts, e.g., changes in SpO2>4% per second and nonphysiological R-peak intervals from the ECG signal were considered artifacts.
Patients were able to see the vital sign measurements during monitoring, but the investigator could only view the measurements once data had been downloaded from the gateway after the end of each patient’s monitoring period. Patients were not required to wear all sensors, but wearing the chest patch was mandatory. Monitoring was initiated prior to discharge and continued at home. Four patients started monitoring after discharge in their own homes; monitoring was initiated between four and 48 hours after discharge. Investigators encouraged patients to wear sensors for at least four days until a maximum of eight days. Patients were contacted daily by phone to check if sensors were still working and to remind them of battery change. In case of questions, patients were able to contact a hotline. In case of monitoring for more than four days, an additional visit was set up to change the chest patch due to battery durability. An investigator visited the patient on the final monitoring day to end the session, talk about experiences of home monitoring, and collect sensors.
Outcomes
The primary outcome was percentage of maximum monitoring time of HR and RR data collected from the chest patch after artifact removal (valid collected data).
Secondary outcomes were:
-
Duration of valid SpO2 and BP monitoring as a percentage of maximum monitoring time
-
Cumulated duration of desaturation as a percentage of maximum monitoring time for the following SpO2 levels: SpO2<88%, SpO2<85%, and SpO2<80%
-
Number of sustained desaturations with SpO2<88% in ≥10 consecutive minutes, and SpO2<85% in ≥5 consecutive minutes
-
Number of sustained deviating vital signs in accordance with predefined thresholds
As an explorative outcome, patients’ experiences and feedback on monitoring at home were examined.
Data analysis
Patients were included in the analysis of patient experiences if sensors were mounted and monitoring of vital signs were initiated. To be included in the analysis of vital signs monitoring, patients needed to be monitored for ≥6 hours after discharge within the first 24 hours. Time of discharge was defined as the time of last early warning score measurement or at the beginning of monitoring just prior to the patient leaving the hospital. Results are reported according to the day of discharge, with day 0 defined as the day of discharge and day 1 being the first full day (24 hours) at home from midnight to midnight, etc.
Duration of monitoring was the total maximum monitoring time per day (1440 minutes for days where patients were monitored 24 hours). Duration of valid monitoring time was defined as the number of minutes where data was collected after artifact removal and periods in which patients were not wearing the sensors. Duration of valid monitoring time was given as a percentage between valid monitoring time and duration of maximum monitoring. Valid monitoring time was calculated both for patients who were intended to wear the sensors and those who actually were monitored by the sensors on the particular day (having valid collected data). Values are presented with median and IQR. IBM SPSS statistics 25.0 was used to perform the analysis.
Results
Participants
406 patients were screened for inclusion and 80 patients gave consent to participate. Main reasons for exclusion were declined consent (n=175), lack of investigator or sensors (n=57), or because the patient was deemed unable to cooperate (n=45) (Figure 1). When comparing patients who declined to participate with those who participated, the proportion of females was 62% vs. 50%; and median age was 70 years (IQR 46–78) vs. 59.5 years (IQR 36–76).
Among included patients 29 patients (36%) were considered healthy without chronic medical diseases prior to hospital admission. Clinical Frailty Scale (CFS)12,13 indicated that most patients managed well. Respiratory disease was the primary reason for acute admission in 56% of included patients (Table 1).
A total of 69 patients were monitored for ≥6 hours after discharge and included in the analysis of monitoring data. These patients did not differ from those who were not included in the analysis of monitoring data.
Monitoring
For each day, the number of patients who wore the sensors is illustrated in Figure 2. For the chest patch and SpO2 sensor >70% of the patients continued to wear the sensors for the full monitoring period. Adherence to wear the BP sensor was 96% on the day of discharge and 50% on day 2.
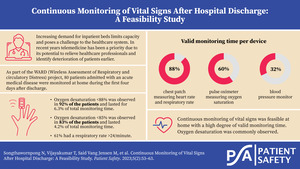
For the full monitoring period, the median percentage of valid monitoring time for patients expected to wear the sensor was 88% (IQR 57%–96%) for the chest patch, 60% (IQR 24%–84%) for the SpO2 sensor, and 32% (IQR 11%–63%) for the BP sensor (Figure 2A).
When considering only patients who actually wore the sensors, the median duration of valid monitoring time was 92% (IQR 79%–99%), 67% (IQR 41%–87%), and 52% (38%–82%) for the chest patch, SpO2 sensor, and BP sensor, respectively (Figure 2B).
Vital signs
The SpO2 sensor was worn by 63 patients. Fifty-eight patients (92%) had at least one episode with SpO2<88%, for a median cumulative duration of 6.3% (IQR 0.9%–22.0%) of the total monitoring time, corresponding to 91 minutes per day. Oxygen desaturation<85% was observed in 52 patients (83%), with a median duration of 4.2% (IQR 0.4%–9.4%) of the monitoring time (34 minutes per day). Thirty-nine patients (62%) had desaturation<80%, with a median cumulative duration of 0.3% (IQR 0%–1.7%) of the monitoring period (four minutes per day).
Sustained deviating vital signs
Occurrence of sustained deviating vital signs are shown in Table 2. Tachypnea with RR>24/min for ≥5 minutes was observed in 42 patients (61%). Bradypnea with RR<5/min and HR>10/min for ≥1 minute was observed in 20 patients (29%), and tachycardia with HR>130/min for ≥30 minutes was observed in 19 patients (28%).
30-days follow-up
Within 30 days of discharge, 20 (25%) patients were readmitted, and none died. Nine (45%) patients had a previous medical history with asthma or COPD, and six (30%) had diabetes mellitus. Six (30%) patients did not have any previous medical history besides the acute reason for admission. Respiratory reasons (AECOPD, pneumonia, or asthma exacerbation) accounted for half of the readmissions (n=11, 55%), and all these patients were also primarily admitted with a respiratory diagnosis. Patients who were readmitted had a median age of 66.5 years (IQR 49–77) compared to 57 years (IQR 35–76) for the patients who were not readmitted. CFS of 5 was observed in 20% of the patients who were readmitted, but only in 5% of the patients who were not.
Feedback from patients
Fifty percent had no complaints regarding wearing the chest patch (Table 3). Forty-two percent had no complaints about the SpO2 sensor, and 19% reported that the SpO2 sensor hindered daily activity, e.g., work or cooking. The BP sensor received the most complaints, and the main complaint was discomfort (48%), with pain associated with inflation of the cuff. Thirty-three percent of patients discontinued BP monitoring on day 1, 50% on day 2, and 54% on day 3 (Figure 2).
Discussion
Continuous monitoring of vital signs at home was feasible. The number of patients who completed the four days monitoring period was high for the chest patch and the SpO2 sensor, but BP monitoring was only achieved in approximately half of the patients. When patients wore the sensors, percentage of time with valid monitoring, after artifact removal, was high (59%–89%). Occurrence of sustained deviating vital signs were high, particularly occurrence of deviating respiratory vital signs.
Monitoring of vital signs
The chest patch was the sensor which most patients wore throughout the monitoring period and collected the most valid monitoring time. Adherence to wearing the SpO2 and BP sensors was poorer, and percentage of valid monitoring time was lower. Duration of valid monitoring time, based on all patients intended to wear the sensors, was comparable to our experiences from in-hospital monitoring.14–17 This was surprising, as patients were free to move around and do everyday activities. An advantage of admission at home and early discharge is that patients can be more physically active18; however, increased activity causes more noise to the signal, and it is described that activity disturbs accuracy of continuous monitoring.19 In a study by Buekers et al., 20 patients wore an SpO2 sensor like the one we used and an activity sensor. They found that up to a third of the collected SpO2 data was considered invalid due to motion artifacts.20 In our study we did not detect that high amount of data loss; however, we have not measured activity, and therefore we cannot rule out whether our patients were less active compared to the study by Buekers et al.
Vital signs
Most patients had periods with sustained deviating vital signs. Particularly, respiratory vital signs deviated often. Almost all patients had periods with oxygen desaturation. Deviating vital signs were expected, as we observed patients during the transition period from acute admission to the first days after discharge. In three previous studies on admitted patients, detection of deviating vital signs was far more frequent when using continuous monitoring compared to spot measurements: In postoperative patients, desaturation <85% was observed in 88% of patients by continuous monitoring compared to only 4% of the patients when using spot measurements.14 In another study, oxygen desaturation <90% was observed four times more frequently by continuous monitoring compared to spot measurements.21 When looking at patients admitted due to AECOPD, desaturation below 80% was observed in 63% of the patients by continuous monitoring, but none of the patients had a comparable low SpO2 registered by spot measurements.15 A part of this discrepancy may be explained by periods without observations, but it has also been shown that manually recorded SpO2 measurements on average have 6.5% higher values than undisturbed continuous monitoring measurements.22 A third of the patients in our study population had no previous medical disease, and even though more than half of the patients were admitted due to a respiratory cause, we were surprised to observe the high occurrence of desaturation and tachypnea. The rate of sustained deviating vital signs was comparable to a previous study from our group based on continuous in-hospital monitoring of patients admitted with AECOPD.15
Bradypnea was observed more frequently (29%) than expected. In the literature, prevalence of sleep apnea in the population is 2%–25%23; the high prevalence of bradypnea episodes and oxygen desaturations may reflect undiagnosed sleep apnea episodes, as we also monitored patients during the night. With this high rate of deviating vital signs, we must ask whether we can trust in our measurements. A previous validation study has shown that our sensors measure HR and SpO2 accurately, whereas measurement of RR had wider limits of agreement (-6 to 7.5/min) than clinically accepted (±3/min).24 We cannot rule out that this imprecision for RR detection may interfere with our results; however, it cannot explain the high occurrence of sustained oxygen desaturation episodes.
Our thresholds for sustained deviating vital signs were based on assumptions made for in-hospital patients, and these thresholds may not be appropriate to transfer to an out-of-hospital setting. Moreover, tachypnea and periods of oxygen desaturation could be signs of ongoing respiratory infection and activity. Further study is needed to clarify an acceptable range of vital signs in patients and healthy subjects who are monitored continuously in a home setting.
30-days follow-up
Continuous monitoring of post-discharge patients may enable early discharge and shorter hospital stays, but deterioration of the primary cause of admission may be undetected. Twenty patients (25%) were readmitted within 30 days after discharge, which is in concordance with previous published data.2–4,25 The most common reason for readmission was respiratory, which could be explained by a worsening of the primary cause of admission. Due to the study design, we were not able to determine whether an intervention during the observation period could have avoided the readmission, but we can infer that a deterioration of vital signs could have been detected earlier and readmission could have been avoided.
Feedback from patients
Adherence to wearing sensors decreased over days and the drop-out rate was most evident for the SpO2 and BP sensors. Despite that half of the patients expressed discomfort after wearing the chest patch, adherence to continued monitoring was high; this was not the case for the BP sensor, where the drop-out rate was >50% on day 3 and most of the patients criticized the BP sensor. We have not found any studies describing compliance in the continuous wearing of a BP sensor or SpO2 sensor, but Downey et al. have previously evaluated a sensor similar to the chest patch during hospitalization, and their experiences were that 82% of the patients found the patch comfortable; however, they had a low response rate (42%) and a drop-out rate of 24%.26 This may indicate an overestimation of positive feedback. Another factor may be that patients in our study wore more sensors and that they were monitored in their own homes, thus increasing the patients’ awareness of the sensors.
Two complaints were dominating: sensors were stressful to wear and were associated with discomfort. With these complaints it is difficult to call the technology “wear-and-forget.”
Strengths and limitations
A strength of our study is that we explored feasibility of continuous monitoring in a home setting in 80 newly discharged patients with an acute medical condition. More than 70% of the patients continued wearing the chest patch and SpO2 sensor for the whole monitoring period, and valid monitoring time was high, which indicated that patients were motivated for wearing the sensors and that the sensors could detect and collect data in a home setting where patients were able to move around.
Our study also comes with limitations. Because only few exclusion criteria were set, the population was very heterogenous. We observed that the study population was younger than those who declined to participate, and it is possible that included patients were more positive about new technology. A selection bias could have been introduced, as the patients were enrolled from the acute medical ward and had short admission time, but 30 days readmission rate was comparable to previously reported readmission rates among medical patients,25 and we consider the population to represent a group of patients who might have a great benefit of home monitoring.
Another limitation was the fact that live transmission of data was not possible. This may affect motivation to continue monitoring, as we were not able to detect if there were any technological problems and we were not able to give patients feedback on their measurements during daily contact. Lack of feedback has been shown to decrease trust of health technology among patients.27 Implementation of live transmission is planned for future studies and is an important step when planning to introduce the technology in a home setting, because a major challenge may be connectivity issues and how we manage to handle alerts from patients monitored in their own homes.11
Conclusion
Monitoring of continuous vital signs was feasible at home with a 59%–89% of valid monitoring time. Adherence to wearing sensors reduced over days, especially for the SpO2 and BP sensors, which could be attributed to discomfort. The lack of live transmission in this study could also affect patients’ motivation. In the transition period after discharge, sustained deviating vital signs were commonly detected, also in patients without chronic medical diseases. Continuous monitoring of vital signs has the potential to improve patient safety by providing crucial information about early deterioration and triggering an intervention; however, defining acceptable vital sign ranges for continuous monitoring at home still needs to be elucidated.
Funding acknowledgements
This work was supported by the Innovation Fund Denmark [8056-00055B], the Capital Region of Denmark’s Research Foundation, Bispebjerg Hospital, Rigshospitalet, and Technical University of Denmark.
No sponsor had any role in the study design, collection of data, analysis, access to the data and interpretation of data, writing, or the decision to submit the article.
Author Contributions
NS: Conceptualization, Methodology, Formal Analysis, Investigation, Writing – Original Draft. TS: Conceptualization, Methodology, Investigation, Writing – Original Draft. MSVJ: Conceptualization, Methodology, Investigation, Writing – Review & Editing. ME: Conceptualization, Methodology, Writing – Review & Editing. HBDS: Conceptualization, Software, Resources, Writing – Review & Editing. EKA: Conceptualization, Methodology, Resources, Writing – Original Draft, Project Administration. CSM: Conceptualization, Methodology, Resources, Writing – Original Draft, Project Administration. VRE: Conceptualization, Methodology, Formal Analysis, Investigation, Writing – Original draft, Project Administration.
Disclosure
Disclosure: CSM, EKA, and HBDS have founded a startup company, WARD247 ApS, to pursue the WARD-projects regulatory and commercial activities. WARD247 ApS has finalized terms for license agreement for any WARD-project software and patents, of which one has been filed. WARD247 ApS has not had any influence on the study design, conduct, analysis, or reporting. CSM and EKA report lecture fees from Radiometer.
About the Authors
Nicharatch Songthawornpong is a medical doctor and research associate at the WARD (Wireless Assessment of Respiratory and circulatory Distress) project and the Department of Anaesthesia and Intensive Care, Copenhagen University Hospital – Bispebjerg and Frederiksberg, Copenhagen, Denmark.
Thivya Vijayakumar is a research associate at the WARD project and the Department of Anaesthesia and Intensive Care, Copenhagen University Hospital – Bispebjerg and Frederiksberg, Copenhagen, Denmark. She will soon graduate as a medical doctor from University of Copenhagen.
Marie Said Vang Jensen is a research associate at the WARD project and the Department of Anaesthesiology, Centre for Cancer and Organ Diseases, Copenhagen University Hospital – Rigshospitalet, Copenhagen, Denmark. She will soon graduate as a medical doctor from University of Copenhagen.
Mikkel Elvekjaer is a medical doctor and holds a Doctor of Philosophy. He is associated with the WARD project and the Department of Anaesthesia and Intensive Care, Copenhagen University Hospital – Bispebjerg and Frederiksberg, Copenhagen, Denmark.
Helge B. D. Sørensen was an associate professor at DTU Health Tech, Technical University of Denmark. His primary area of research was biomedical signal processing. He founded the WARD project on artificial intelligence–supported wireless vital sign monitoring together with Christian S. Mayhoff and Eske K. Aasvang.
Eske K. Aasvang is a professor at the Department of Anaesthesiology, Centre for Cancer and Organ Diseases, Copenhagen University Hospital – Rigshospitalet, Copenhagen, Denmark. His primary research area is perioperative medicine and enhanced recovery after surgery. He founded the WARD project on artificial intelligence–supported wireless vital sign monitoring together with Christian S. Mayhoff and Helge B. D. Sørensen.
Christian S. Meyhoff is a professor at the Department of Anaesthesia and Intensive Care, Copenhagen University Hospital – Bispebjerg and Frederiksberg, Copenhagen, Denmark. His primary research area is prevention of postoperative cardiopulmonary complications and large randomized controlled trials. He founded the WARD project on artificial intelligence–supported wireless vital sign monitoring together with Eske K. Aasvang and Helge B. D. Sørensen.
Vibeke R. Eriksen is a medical doctor and holds a Doctor of Philosophy. She is associated with the WARD project in a postdoc position and to the Department of Anaesthesiology, Centre for Cancer and Organ Diseases, Copenhagen University Hospital – Rigshospitalet, Copenhagen.