Introduction
In late 2017, the United States declared the opioid epidemic “a public health emergency.”1 Opioid-related deaths have quadrupled in the United States over the past 15 years and have surpassed motor vehicle accidents as the leading cause of injury-related deaths.2–4 In addition to claimed lives, this problem has created a considerable economic strain. The estimated total cost burden of the opioid epidemic (accounting for overdose, treatment, and dependence) was estimated at 78.5 billion U.S. dollars.5 A quarter of this economic burden was funded by public sources such as Medicaid, Medicare, and government substance abuse programs.5 This epidemic costs the United States lives and dollars, and has garnered the attention of a concerned public and the healthcare community.
The increase in the number of opioid prescriptions correlates with opioid abuse-related deaths.2,3,6,7 The literature considers the reason for increased physician opioid prescribing patterns as multifactorial. Some sources consider that the classification of pain as “the fifth vital sign” in the 1990s spurred physicians to increase quantities of prescribed opioids in order to control pain and achieve patient satisfaction.3,8 Another proposed factor is that learned prescribing patterns from peers and personal experience lead to behaviors that err toward prescribing too much rather than too little.8
Operating rooms have been claimed as “unintended gateways” to the opioid epidemic by the media9 and it could be inferred that the surgeons writing the prescriptions are the gatekeepers. If so, surgeons play a unique role in addressing the opioid epidemic. An excess of prescribed opioid analgesics is commonly reported in postsurgical care.10,11 The area of postoperative pain management provides a twofold area of interest.
First, surgery is often a patient’s introduction to opioids and offers these opioid-naive patients access to potentially addictive substances.10 The prescription of these medications increases the probability of the patient developing into a chronic opioid abuser.2
Secondly, and of most interest to the quality initiative herein, many patients never finish all the prescribed analgesic medication in the postoperative period.11 Overprescribing, in addition to failure to properly dispose of the leftover drugs, has increased the prevalence of pill diversion. Pill diversion is the inappropriate access to and use of drugs by a person other than to whom they were prescribed. Over two-thirds of opioid abusers receive their medications in this manner.2 By prescribing pills to patients who either never start the medication or fail to complete a large prescription, surgeons unintentionally contribute to a potential diversion pool of drugs, opioid misuse and abuse, and opioid-related deaths.
This study evaluates postoperative pain medication prescribing and consumption patterns for many common surgical procedures. The primary objective was to measure our potential diversion pool using the parameters of prescription and consumption rates of opioids, and correlating them to patients’ consumption of opioids in the last 24 hours of their inpatient stay. By measuring these parameters, we hope to achieve our second objective, which was to set the groundwork for an institutional postoperative prescribing guideline for opioid medications to address patient needs without providing excessive numbers of pills to our community.
Our hypothesis was that a proportion of the prescribed opioids were going unused and potentially being added to the diversion pool. In addition, we hypothesized that there was a relationship between the number of opioids used in the last 24 hours of inpatient stay and how many are consumed as an outpatient. The goal was to standardize the manner in which our house staff and attendings evaluate a patient’s needs for pain management postoperatively by consideration of the procedure, the last 24 hours of inpatient opioid use, and the individual patient. The final objective was to assess long-term follow-up data regarding opioid prescribing patterns.
Methods
This study was approved by the Institutional Review Board of Thomas Jefferson University. Initial data collection consisted of three separate surveys: a survey of surgery residents’ opioid prescribing practice, a survey of surgery attendings’ opioid prescribing practice, and a telephone survey of patients operated on between August 2017 and March 2018 at the Thomas Jefferson University Hospital. The surgery resident survey included three questions (Table 1).
The attending survey included 11 questions to determine attitudes about the opioid epidemic and our department’s practices and potential contribution to the epidemic (Table 2).
The patient telephone survey data were maintained in a REDcap database. Demographic data and inpatient data, including operation, length of stay, and postoperative opioid requirements, were entered and patients were subsequently contacted. Patients were excluded if they were found to be opioid tolerant preoperatively, were discharged to a rehabilitation facility, were incarcerated postoperatively, or could not answer key survey questions.
Procedures included were as follows: laparoscopic cholecystectomy, laparoscopic and open hernia repair, video-assisted thoracoscopic lung resection, laparoscopic and open colectomy, mastectomy, proximal and distal pancreatectomy, esophagectomy and gastrectomy, aortic bypass, kidney transplant, and liver resection. Thirty patients from each surgery type were set as the target to obtain a representative sample. Patients were asked to retrieve their prescription bottles and were asked the following eight questions (Table 3).
The mean, median, and range of prescribed and consumed pills for each procedure were determined. The standard deviation for consumed pills for each procedure was also determined. The “potential diversion pool” for the department was calculated (pills prescribed minus pills consumed). The relation between the number of tablets required in the last 24 hours of the patient’s in-hospital stay and the number of tablets required after discharge was determined overall and for each procedure.
The data from the survey was then used to create a Thomas Jefferson University Hospitals Inc. (TJUH Inc.) Opioid Toolkit, which included an opioid prescribing guideline for our surgery department. This toolkit consisted of a list of procedures and the recommended dosing alongside a modifier based on the number of opioids consumed in the patient’s last 24 hours of inpatient usage. Additionally, the toolkit contained links to opioid prescribing literature and resources, and was available to every clinical provider on the intranet homepage of all hospitals within the system. Additional work was conducted alongside the electronic medical record (EMR) team to change the default number of opioids prescribed when ordered from 20 pills to 10 pills and from seven to three days. This process change was carried out across the multi-institution health system and results were recorded.
Follow-up data for the years after the initiation of the TJUH Inc. Opioid Toolkit were tracked and recorded.
Results
Resident Survey Data
The results of the resident survey are displayed in Table 4. These results reveal that there was no standard number of pills prescribed for any procedure and that the number of pills individual residents prescribed varied greatly (e.g., between 10 and 50 pills for laparoscopic colon resection). In addition, the survey revealed that very few residents predicted that patients are using 100% of their prescriptions. Most residents predicted that patients are using 50% of their prescriptions or less. The final question revealed that the reasoning for this prescribing behavior varied: There is continued behavior from early training (45%), lack of self-perceived knowledge of what patients need for certain procedures (41%), lack of ability to predict which patients need more (52%), a concern that patients will have to call the attending for refills (62%), and a need to complete discharge workflow (18%).
Attending Survey Data
The surgery attending survey asked 12 questions, listed in Table 5. The answers revealed that attendings agreed there is a nationwide problem with opioid overprescribing for postoperative patients (84%), but many were not sure if there was an overprescribing problem within our department (51%). Many believed that their patients do not have opioid pain pills unused (42%) and that no one other than their patients use the pills that they prescribe (46%). Very few attendings agreed that they screen for signs or symptoms of addiction or withdrawal postoperatively (31%) and even fewer educated patients on how to dispose of leftover opioid pain pills (6%). The majority of attendings felt that patient satisfaction scores could be adversely affected (70%) and pain control could be inadequate for some patients (56%) if postoperative pain prescriptions were decreased. Attendings agreed that a guideline for prescribing postoperative opioids for attendings (95%) and residents (93%) would be helpful.
Patient Survey Data
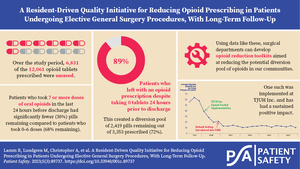
For our 357 patients, there were 12,061 pills prescribed and 5,320 were consumed, leaving a potential diversion pool of 6,831 pills. The data in Table 6 display the mean, median, and range of pills prescribed and consumed for each procedure. For every procedure, the range of tablets prescribed varied greatly, and the mean and median consumed were low relative to the mean prescribed.
The data in Table 7 display the potential diversion pool, with subgroups by the procedure. Every procedure group, other than vascular bypass, mastectomy, and liver resection, had 50% or more of the total pills unused. The potential diversion pool for each procedure is the number in the “Unused” column.
Relation to Final 24 Hours of Inpatient Opioid Use
The mean and median prescribed provided a general guideline for an appropriate number of pills to prescribe per procedure; however, this did not provide guidance on what to prescribe per patient undergoing each procedure. To individualize the number of doses prescribed to each individual patient, the last 24 hours of inpatient need for opioids were analyzed. These data are displayed in Table 8. Of note, data from 33 patients were unable to be obtained in regards to the last 24 hours of inpatient opioid use due to documentation error, so they were omitted from Table 8. At our institution, the most common postoperative opioid order includes oxycodone 5 milligrams every four hours as needed for moderate pain. The patient has the option to receive extra doses as needed for severe pain. If the patient does not ask for an extra dose, they will receive between zero and six pills total in 24 hours. The data reveal that patients who take pain medicine as prescribed or less (0–6 tablets in 24 hours) take far less of their prescription after they get home, compared to patients who take more pills in the last 24 hours of hospitalization (7 or more pills). This relationship remains for both laparoscopic and open cases and holds for all individual procedures.
Patient Request for Opioid Refills
There was concern in the attending survey related to patient need to call for refills. The call rate for refills in the minimally invasive group was 8% and the call rate for refills in the open group was 17%.
Opioid Prescription Rates
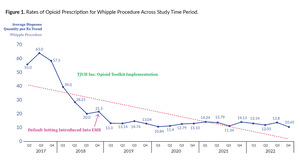
Figures 1 and 2 show representative images of opioid prescription rates for two select procedures (Whipple procedure and inguinal hernia repair) and denote the time when the toolkit and the EMR changes took place. For reference, the Whipple procedure is anatomically a pancreaticoduodenectomy, which involves removing a portion of the pancreas and duodenum as well as recreating the connection between the remainder of the pancreas and the bowel. Of note, two distinct time points are displayed on the graphs. The first is the implementation of the TJUH Inc. Opioid Toolkit and the second is the default setting change in the EMR which lowered the number of auto-populated pills for each prescription. These two interventions were distinctly implemented to isolate the effect of each.
Based on the data collected, this study compiled a recommended prescription based on each procedure, which is displayed in Table 9. These are the current recommendations embedded in our EMR Record Toolkit.
Discussion
This study was a retrospective review with a purpose of informing the attitudes and practices surrounding postoperative opioid prescriptions at our institution. The data collected in this study confirm the data collected by the other institutions that have performed opioid prescribing studies. This study showed that two distinct interventions (implementation of the opioid reduction toolkit, and lowering the default number of pills) had a significant, compound, and lasting effect on decreasing opioid prescription use in the institution.
Based on survey data obtained from residents and attendings, several issues which informed the ultimate toolkit intervention were highlighted. Most attending and resident respondents agreed there was a nationwide issue with overprescribing opioids, but were unsure whether it existed within the department. Many believed their own patients did not have opioid pills that went unused, did not screen for signs of diversion themselves, and expressed worry that decreasing the number of pills prescribed would increase pain for their patients. These beliefs illustrated a real need for data evaluation, as well as filled a gap for the clinical providers in terms of what the actual trends were amongst their patients. This set the culture that became accepting of the toolkit itself.
Chiu et al., in an anonymous online survey given to junior and senior residents at a single institution, found that not all surgical trainees were given formal training in regard to appropriate opioid prescribing, and prescription patterns varied widely among the group. Specifically, those that were not trained had a tendency to overprescribe.12 Blay et al. found that among resident trainees, attending surgeons, and advanced care providers there was wide variation in opioid prescription patterns when looking at both numbers of tablets prescribed as well as total milligram equivalents.13
Hill et al. evaluated the number of opioid pills taken at home after discharge and found that the number of pills consumed was associated with patient age and the number of medications taken before discharge, but not the type of surgery performed.2 Further, the number of opioid medications prescribed varied widely by operation, and only 28% of the total prescribed pills were consumed.3 The authors generated operation-specific pill recommendations that would satisfy 80% of patients undergoing the procedures evaluated.3
Scully et al. evaluated the effectiveness of opioid prescribing methods by analyzing refill rates instead of pills consumed, and proposed procedure-specific optimal discharge prescription lengths.3,6 These studies and more illustrate the need to properly optimize postoperative opioid prescribing guidelines in order to reduce excess opioids in our community.
One concern which was elucidated in the survey, from both residents and attendings, was the rate in which patients would call back to the office requesting a refill. The study found that in the minimally invasive and open group, the rate of phone calls for refills was 8% and 17%, respectively. Amongst the literature there appears to be a wide range of refill request rates, with some studies reporting as high as 25% for procedures such as tonsillectomy.14 Others which focus on general, colorectal, vascular, and gynecologic surgical procedures reported a rate ranging from 3%–6% of refill requests.15 Clearly, additional studies need to be performed in order to match the correct patient with the correct initial prescription of opioids following surgery.
Finally, changes to our EMR which decrease the default number of prescribed opioids had a profound impact on lowering the number of opioids prescribed for all procedures. Delgado et al. showed changing the default opioids prescribed in the EMR significantly lowered the number of pills prescribed in two tertiary emergency rooms.16 This effect can and should be harnessed to tailor prescribing patterns with opioids and other controlled substances. The effect of this intervention combined with the toolkit led to additive reduction in the number of opioids prescribed in our study. This synergistic effect of toolkits plus EMR default lowering has been previously reported by multiple corroborating studies.17–19 The authors were pleased to see that current data (Figures 1 and 2) illustrate a sustained reduction in opioid prescribing over time.
Our study has several important limitations. It is a single-institution study and surveys of the residents and attendings were partially qualitative opinion surveys. The results of the telephone survey (other than those confirmed in the electronic record) are patient self-reported. Patients who did not still have their prescription bottles at home had to recall the number of tablets they consumed. Most patients had a very easy time remembering how many tablets they consumed. The patients who could not recall were excluded. The procedures included and the number of patients in each group was limited by the number of procedures performed in the preceding months, the number of patients who responded to our telephone outreach, and the number of exclusions. Therefore, our “n” for individual procedures did not always reach the desired number of 30. Additionally, we chose to count the number of pills taken rather than calculate morphine equivalents. The rationale for evaluating this study based on pills rather than morphine equivalents was made since this was a provider- and patient-facing study. As most providers utilize number of pills as their prescribing method, as opposed to calculating the morphine equivalents, this was chosen as the unit of study.
Despite the limitations of the study, the benefits of the data are numerous. The data align with what has previously been published—including Hill et al.'s guidelines for opioid prescriptions for general surgery procedures. We feel confident with making recommendations to our surgeons and surgery residents on a mean number of tablets to prescribe at discharge for the included procedures. These recommendations have decreased current prescribing practices by 50% or more for each procedure, reducing the potential diversion pool created by our department by thousands of pills annually.
A 2017 JAMA Surgery article studied the correlation between 24-hour predischarge opioid use and the number of opioids prescribed at hospital discharge. The authors found 6,548 patients out of 21,452 (35.7%) used no opioids in the 24 hours prior to hospital discharge and yet 2,988 (45.6%) were sent home with opioids.12 Similarly, we found that 100% of patients who took zero pills in the 24 hours prior to discharge were given opioid prescriptions on discharge. The diversion pool in this patient population was the highest for all procedures.
With current electronic prescribing capabilities, the need for refills should not hinder an institution’s plans to optimize (decrease) opioid prescribing practices. Additional studies are needed to track compliance with guidelines and the need for refills, and determine best practices for treating more complex patient populations, including emergent cases, postoperative complications, and the opioid tolerant.
Acknowledgements
The authors would like to acknowledge the residents, advanced practice providers, faculty, nursing staff, and Thomas Jefferson University Hospital for supporting this project.
Disclosure
The authors declare that they have no relevant or material financial interests.
About the Authors
Ryan Lamm (ryan.lamm@jefferson.edu) is a current chief resident in general surgery at Thomas Jefferson University with a special interest in surgical oncology and quality and safety. He will be pursuing a fellowship in surgical oncology at City of Hope in California next year.
Megan Lundgren is a former general surgery resident from Thomas Jefferson University who worked extensively on this project and currently practices at Penn Highlands in minimally invasive, bariatric, and general surgery in DuBois, Pennsylvania.
Adrienne Christopher is a former general surgery resident from Thomas Jefferson University who is currently a plastic surgery resident at Vanderbilt University.
Jacob Woodroof is a current general surgery resident at Thomas Jefferson University.
Lindsay Edwards is a current general surgery resident at Thomas Jefferson University.
Christopher Kustera is a former medical student at Sidney Kimmel Medical College and a current emergency medicine resident at St. Luke’s in Bethlehem, Pennsylvania.
Charles J. Yeo is the Samuel D. Gross Professor and Chair of Surgery at Thomas Jefferson University. Additionally, he is the senior vice president and chair of Enterprise Surgery for Jefferson Health and a co-director of the Pancreas, Biliary & Related Cancer Center at Thomas Jefferson University Hospital. His support enabled this project to be undertaken.
Kristin M. Noonan is a clinical associate professor and the director of Surgical Quality and Safety at Jefferson Abington Hospital. She is the current program director of the general surgery residency at Jefferson Abington Hospital.
Harish Lavu is a professor of surgery at Thomas Jefferson University and co-vice chair for Quality.
Caitlyn Costanzo is an assistant professor of surgery at Thomas Jefferson University and co-vice chair for Quality. Additionally, she is the colorectal fellowship program director.
Scott Cowan is the medical director for Enterprise Risk, interim chief quality officer for Jefferson Health, and the Jefferson enterprise lead for Surgery Quality and Safety. He received his doctor of medicine degree at Thomas Jefferson Medical College in 1997 and then completed a seven-year surgery residency at Thomas Jefferson University Hospital. Dr. Cowan completed his cardiothoracic surgery fellowship at Massachusetts General Hospital in 2007 and worked for three years in the University of Pennsylvania Health System as a general thoracic surgeon. He has been a faculty member at Thomas Jefferson Health System for over 10 years and holds the rank of associate professor of Surgery. Dr. Cowan is a frequent speaker and has more than 70 peer-reviewed publications in surgery and quality and safety–related journals.