Patient safety in Pennsylvania is largely directed by Pennsylvania’s Medical Care Availability and Reduction of Error (MCARE) Act, which requires some healthcare organizations to report incidents, serious events, and infrastructure failures (see MCARE for definitions) into the Pennsylvania Patient Safety Reporting System (PA-PSRS).1 Many variables, including the complexity of healthcare, impact reporting and create inconsistencies.
This is most evident in facility determination of serious events. Final Guidance2 developed by key stakeholders provides criteria for serious event classification, with the intent to create consistency in reporting among facilities. The PSA encourages the use of the Final Guidance, as well as the FAQs and Reporting Decision3 Tree, to help make determinations in reporting.
A recent review of PA-PSRS events related to returns to surgery uncovered opportunities for reinforcement related to two specific guidance principles, known complications and high risk.
The PSA found that many return to surgery events are reported as incidents. If you perform surgical procedures in your area, consider reviewing the Final Guidance, #3 and #4.
1. Known Complications (FG3)[1]
A known complication is not typically considered anticipated by the patient.
If a patient experiences a “known complication” and/or a situation that is “discussed during consent,” the event is likely still reportable as a serious event if no other exclusion criteria apply, such as the patient being a higher-than-normal risk.
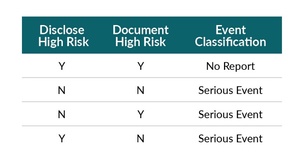
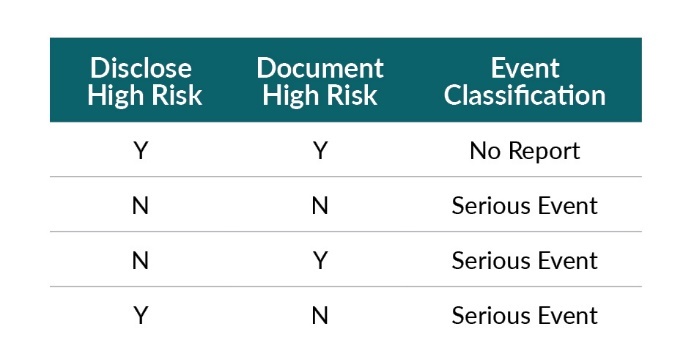
2. High Risk (FG4)[2]
If a patient is at “high risk” for a return to surgery, it would be considered anticipated and not reportable as a serious event or incident. There is no need to enter a report into PA-PSRS.
Two conditions must be met for a patient to meet high-risk criteria.
a. Disclosure to the patient (of the high probability of complication)
b. Documentation in the chart/consent (of the high probability of complication)
*This does NOT pertain to the “standard” consent discussion or document (see #1 above).
Most return to surgery events should be reported into PA-PSRS as serious events. Nothing in healthcare is absolute and there are many nuances that make each situation unique which may impact the report classification. Please contact your facility’s patient safety liaison for additional support.
Disclosure
The author declares that they have no relevant or material financial interests.
This article was previously distributed in the February 15, 2023, educational email of the Patient Safety Authority, available at https://conta.cc/3S2Qqfe.
About the Author
Patient Safety Authority (patientsafety.pa.gov) is an independent state agency that oversees the Pennsylvania Patient Safety Reporting System (PA-PSRS), the largest database of its kind in the United States.
“The disclosure of a potential complication on a patient consent form does not, in itself, constitute anticipation of the complication by the patient.”2
“Complications may be considered anticipated (and therefore not meeting the Serious Event definition) when they occur frequently or the risk of the complication is considered high for a particular patient and the high probability of this complication was disclosed to the patient in the informed consent discussion and documented either on the consent form or medical record.”2